Abstract
In the domestic cat, morula-blastocyst formation in vitro is compromised after intracytoplasmic sperm injection (ICSI) with testicular compared to ejaculated spermatozoa. The aim of this study was to determine the cellular basis of the lower developmental potential of testicular spermatozoa. Specifically, we examined the influence of sperm DNA fragmentation (evaluated by TUNEL assay) and centrosomal function (assessed by sperm aster formation after ICSI) on first-cleavage timing, developmental rate, and morula-blastocyst formation. Because the incidences of DNA fragmentation were not different between testicular and ejaculated sperm suspensions, DNA integrity was not the origin of the reduced developmental potential of testicular spermatozoa. After ICSI, proportions of fertilized and cleaved oocytes were similar and not influenced by sperm source. However, observations made at 5 h post-activation clearly demonstrated that 1) zygotes generally contained a large sperm aster after ICSI with ejaculated spermatozoa, a phenomenon never observed with testicular spermatozoa, and 2) proportions of zygotes with short or absent sperm asters were higher after ICSI with testicular spermatozoa than using ejaculated spermatozoa. The poor pattern of aster formation arose from the testicular sperm centrosome, which contributed to a delayed first cleavage, a slower developmental rate, and a reduced formation of morulae and blastocysts compared to ejaculated spermatozoa. When a testicular sperm centrosome was replaced by a centrosome from an ejaculated spermatozoon, kinetics of first cell cycle as well as embryo development quality significantly improved and were comparable to data from ejaculated spermatozoa. Results demonstrate for the first time in mammals that maturity of the cat sperm centrosome (likely via epididymal transit) contributes to an enhanced ability of the spermatozoon to produce embryos that develop normally to the morula and blastocyst stages.
Keywords: assisted reproductive technology, early development, gamete biology, sperm maturation
INTRODUCTION
Intracytoplasmic sperm injection (ICSI) with testicular spermatozoa offers many advantages. After unexpected death or castration for medical reasons, this technique allows the study and use of immotile intragonadal gametes from genetically valuable 1) adult males with obstructive and nonobstructive azoospermia and 2) prepubertal males after xenografting of testis tissue in immunodeficient mice [1]. Our laboratory is currently examining these emerging technologies in the domestic cat as a model for developing strategies to genetically manage rare biomedical models as well as to conserve the 36 wild species in the family Felidae [2]. It is well established that ejaculated or epididymal cat spermatozoa fertilize conspecific oocytes after ICSI, which can lead to the formation of high-quality embryos [3–5] and live young after transfer [4]. Recent results have revealed that cat spermatozoa from the ejaculate as well as from the testis have identical fertilizing abilities with resulting embryos having equivalent capacity to develop to the 8–16-cell stage in vitro [6]. Regardless of the sperm source, morula and blastocyst formations were not resulting from parthenogenetic activations [6]. However, ICSI with testicular spermatozoa produces more embryos that appear to be afflicted with a block before the morula-blastocyst stage [6]. This difference in the ability to induce normal embryo development (or developmental potential) between intact testicular and ejaculated spermatozoa has not been reported in other species [7–9].
The incidence of sperm DNA fragmentation appears to be a useful predictor of successful human embryo development after ICSI [10–12]. However, incidence of sperm DNA fragmentation has no adverse consequences in the early embryo cleavages, specifically before the morula-blastocyst formation [13]. The impact of sperm DNA fragmentation on the embryo development after ICSI has not been studied in the domestic cat. As in other species, assembling of a compacted sperm nucleus, acrosome, midpiece, and flagellum occurs in the cat as the spermatid transforms into a spermatozoon [14, 15]. Epididymal transit then creates a microenvironment that helps to convert immotile, immature testicular spermatozoa into fully fertile, competent cells [16, 17]. Main morphological conversions occurring during transit in the cat epididymis include cytoplasmic droplet migration [16], as well as sperm chromatin condensation and stabilization through the formation of disulfide bonds [18]. Thus, prior to epididymal transit, cat sperm DNA appear more vulnerable to induced denaturation [18] and, therefore, may be more prone to fragmentations. Additionally, because it is believed that epididymal transit also serves to eliminate defective cat spermatozoa [19], it seems reasonable to expect a higher incidence of DNA fragmentation in testicular sperm nuclei. It already has been demonstrated that chromatin integrity in ejaculated spermatozoa from normo-spermic versus teratospermic domestic cats has no influence on fertilization and early embryo cleavage after ICSI [20]. However, the status and impact of DNA fragmentation in testicular (versus ejaculated) spermatozoa has never been examined in any felid species.
Another paternally inherited component of embryo development is the sperm centrosome, which is responsible for aster assembling during the first cell cycle. Except in rodents, centrosomal constituents are reciprocally reduced during gametogenesis so that their components complement each other after fertilization, forming a functional biparental zygotic centrosome [21]. Proper sperm aster formation is crucial for pronuclear migration and apposition as well as mitotic spindle formation [15, 22, 23]. A defect in sperm aster formation can have serious consequences on subsequent embryo cleavages, possibly resulting in multiple mitotic spindles, disorganized chromosomes, or improper cell divisions [24–26]. Furthermore, in the bovine, a large-sized sperm aster in the cytoplasm of fertilized oocytes is positively correlated to subsequent morula and blastocyst formations [23]. However, no information is available on centrosomal functions of cat testicular versus ejaculated spermatozoa during the zygotic cycle as well as its impact on subsequent embryo cleavages and morula-blastocyst formation. Using the domestic cat as a model, the purpose of this study was to explore the cellular etiology to the compromised developmental potential of felid testicular spermatozoa. Specifically, investigation focused on examining the influence of DNA fragmentation and centrosomal functions on embryo development in vitro after ICSI with testicular compared to ejaculated spermatozoa.
MATERIALS AND METHODS
Gamete Collections and Preparations
Ovaries and testes from adult domestic cats were harvested at local veterinary clinics and transported in PBS at 4°C to the laboratory within 6 h of routine ovario-hysterectomy or orchiectomy. Grade I immature oocytes were collected after slicing the ovaries and then were cultured in in vitro maturation medium for 28 h as previously reported [27]. There were two sources of sperm, one from within the testis and the other from previously collected and cryopreserved ejaculates [6]. For isolation of testicular spermatozoa, testes from 18 males (one male/replicate; nine replicates in experiment 1, nine replicates in experiment 2) were dissected in 500 μl Hepes-Ham F10 medium (Irvine Scientific, Santa Ana, CA) supplemented with 1.0 mM pyruvate, 2.0 mM glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 5% fetal calf serum at room temperature (complete Hepes-Ham). The testicular cell suspension from each male then was centrifuged (300 × g, 8 min) and the pellet suspended in 30 μl of complete Hepes-Ham. For ejaculated spermatozoa, frozen/thawed ejaculates from three proven breeder males (one ejaculate/ replicate; nine replicates in experiment 1, nine replicates in experiment 2) were alternatively used. After thawing, motile ejaculated spermatozoa were selected by swim-up processing for 30 min in complete Hepes-Ham [27]. In experiment 2, tubes containing 500 μl of testicular or ejaculated sperm suspension were transferred to a bath containing ice water (0°C) and were sonicated five times for 3 sec with 5-sec pauses at setting 3 of a 60 Sonic Dismembrator (Fisher Scientific, Pittsburgh, PA). This treatment resulted in separation of heads and midpieces in more than 95% of spermatozoa.
Evaluation of Sperm DNA Fragmentation
Sperm DNA fragmentation was evaluated in sperm preparations used for ICSI (nine samples of testicular versus nine samples of ejaculated spermatozoa in experiment 1; nine samples of sonicated testicular versus nine samples of sonicated ejaculated spermatozoa in experiment 2) using the TUNEL assay (Roche Diagnostic, Indianapolis, IN). Samples of each sperm preparation (10 μl) were smeared on microscope slides, fixed in 4% paraformaldehyde for 1 h at room temperature, and permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate for 2 min on ice. The remainder of the procedure was conducted according to the manufacturer instructions. Briefly, samples were incubated in TUNEL reaction mixture in the dark at 38.5°C for 1 h followed by evaluation under a microscope fitted with epifluorescence (Olympus BX 41; Olympus Corporation, Melville, NY). Additional slides were used for the negative (no enzyme terminal transferase) and positive (using 1 mg/ml DNAseI) controls. Four hundred spermatozoa were randomly analyzed per sample to determine the percentage of TUNEL-positive spermatozoa.
Immunostaining of Sperm Centrioles
To reveal the presence of centrosomes by staining centriole pairs in testicular and ejaculated sperm populations (nine samples of testicular spermatozoa and nine samples of ejaculated spermatozoa in experiment 1) and to validate the separation of the centrosome from the sperm head after sonication (nine samples of sonicated testicular spermatozoa and nine samples of sonicated ejaculated spermatozoa in experiment 2), 10 μl of each sperm suspension were smeared on a glass slide and fixed in methanol (−20°C for 10 min). Fixed cells were then incubated with a monoclonal anti-centrin antibody (1/1000; generous gift from Dr. Jeffrey Salisbury) in PBS for 1 h at 38.5°C. After three washings (15 min each) in PBS, fixed spermatozoa were incubated with a second anti-mouse IgG labeled with FITC (Sigma Chemical Co., St. Louis, MO) diluted 1/250 for 1 h at 38.5°C. Sperm chromatin then was stained with Hoechst 33342 (1 μg/ml; Sigma) before observation under a microscope fitted with epifluorescence (Olympus BX 41).
ICSI Procedure
In experiment 1, 5 μl of testicular sperm suspension (from each of the nine males) or thawed ejaculated spermatozoa (from one of the three breeders) were mixed with an equal volume of 10% polyvinylpyrrolidone (PVP; Irvine Scientific). The two different sperm drops were placed separately in the center of a 50×9-mm Petri dish and surrounded with four 10-μl drops of complete Hepes-Ham containing in vitro matured oocytes (previously denuded by gentle pipetting in 0.2% hyaluronidase; Sigma). The dish was flooded with mineral oil and maintained on the heated stage (38.5°C) of an inverted microscope (Olympus IX 70) equipped with micromanipulators (Narishige, Sterling, VA) and holding and microinjection pipettes (Humagen Fertility Diagnostics, Inc., Charlottesville, VA). A single morphologically normal spermatozoon was selected from a sperm drop (motile ejaculated spermatozoon was immobilized by drawing the injection pipette across the midpiece), aspirated, and injected into an oocyte with a visible first polar body [6]. In experiment 2, 5 μl of sonicated testicular and ejaculated sperm suspensions were mixed with 10% PVP and placed separately in the Petri dish. A single testicular or ejaculated sperm head was carefully positioned proximal to a single testicular or ejaculated midpiece (Fig. 1A), gently loaded in the micropipette (Fig. 1B), then injected and carefully deposited into an oocyte with a visible first polar body (Fig. 1, C and D). In experiments 1 and 2, oocytes were activated after ICSI by incubation in complete Hepes-Ham containing 7% ethanol for 5 min at 38.5°C [6]. After extensive rinsing, injected oocytes were cultured in vitro in complete Ham without Hepes (38.5°C, 5% CO2 in air). In experiments 1 and 2, additional oocytes were sham injected (no sperm) to produce parthenogenetic controls (see Experimental Design and Statistical Analysis for oocyte numbers per treatment group).
FIG. 1.
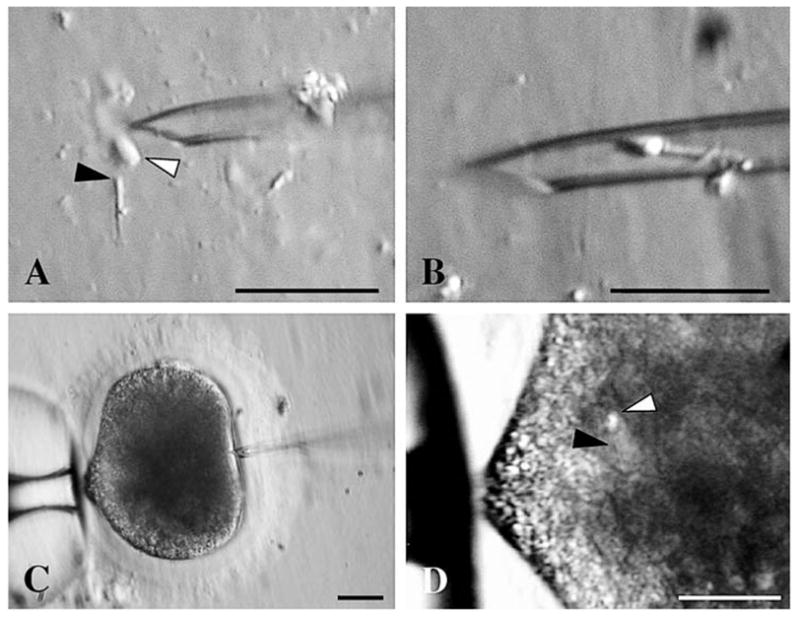
ICSI procedure with sonicated spermatozoa. A) Orientation of a single testicular or ejaculated sperm head (white arrow) proximal to a single testicular or ejaculated midpiece (black arrow). B) Loading of the combination in the ICSI micropipette. C) Sperm head and midpiece maintained at the tip of the ICSI pipette before the injection into an oocyte with a visible first polar body. D) Sperm head (white arrow) and midpiece (black arrow) deposited into the oocyte cytoplasm. Bar = 20 μm.
Immunostaining of Presumptive Zygotes
In experiments 1 and 2, presumptive zygotes as well as parthenogenetic controls were fixed at 5 h postactivation (hpa) in 2.5% paraformaldehyde for 30 min at 38.5°C. After three washings in PBS, the nonspecific antigenic sites were saturated with a solution of PBS containing 0.5% Triton X-100 and 20% FCS for 30 min at 38.5°C. Oocytes were then incubated overnight at 4°C with anti-α-tubulin and anti-β-tubulin monoclonal antibodies (1/1000; Sigma) in PBS containing 0.5% Triton X-100 and 2% FCS. After three washings (15 min each) in PBS, oocytes were incubated with a FITC-labeled anti-mouse IgG (Sigma) diluted 1/150 for 1 h at 38.5°C. Chromatin was then stained with Hoechst 33342 (1 μg/ml) in PBS for 5 min at 38.5°C. Oocytes were mounted on ring Teflon slides with Vectashield medium (Vector Laboratories, Burlingame, CA) under coverslips sealed with nail polish. Oocytes then were observed with a microscope fitted with epifluorescence (Olympus BX 41) and with a confocal microscope (LSM 510, Carl Zeiss Inc., Drive Thornwood, NY) to evaluate dimensions of male pronuclei, analyze microtubule organization and chromatin structures, and capture images.
Experimental Design and Statistical Analysis
Experiment 1 was designed to compare 1) the incidence of DNA fragmentations and centrosomal functions of testicular versus ejaculated spermatozoa and 2) their associations with the success of the embryo development in vitro. Experiment 2 was inspired by experiment 1 results and was designed to demonstrate the impact of the centrosome source on embryo development in vitro. After sonication of the sperm suspensions, oocytes were microinjected with four different combinations of sperm heads (testicular or ejaculated) and centrosomes (testicular or ejaculated). In each of the nine replicates of experiments 1 and 2, sperm suspensions were assessed for DNA fragmentation and presence of centrioles. Grade I immature oocytes from different ovaries of random females were pooled and cultured for in vitro maturation yielding ~90% of nuclear maturation, which is similar to the percentages measured in other contemporary studies from our laboratory [6, 27]. Oocytes then were randomly and equally distributed among different ICSI treatments (in experiment 1, n = 315 total injected oocytes with testicular spermatozoa [35 injected oocytes ×9 replicates], n =315 total injected oocytes in parallel with ejaculated spermatozoa [35 × 9 replicates], n = 90 total sham-injected oocytes [10 × 9 replicates]; in experiment 2, n = 315 total injected oocytes [35 × 9 replicates] with each combination of testicular or ejaculated sperm head and testicular or ejaculated midpiece, n = 90 total sham-injected oocytes [10 × 9 replicates]). Experiments were replicated over different days (one replicate per day) with different batches of oocytes. In each treatment group, half of the oocytes injected with spermatozoa (and all parthenogenetic controls) were fixed and stained at 5 hpa to assess the percentage of fertilization after ICSI (number of oocytes with two pronuclei relative to total number of injected oocytes) and the percentage of activation after sham ICSI (number of oocytes with pronucleus relative to total number of sham-injected oocytes). Pattern of sperm aster formation was evaluated and defined as the proportion of oocytes with a large, short, or absent sperm aster relative to total number of fertilized oocytes. Nuclear status of unfertilized oocytes at 5 hpa also was recorded. The other half of oocytes injected with spermatozoa remained in culture. Percentages of two-cell embryos were recorded every hour from 20 to 32 hpa. The effect of time postactivation on the percentage of two-cell embryos was expressed by a linear regression (SigmaStat; SPSS, Chicago, IL). Mean times of first cleavage (calculated from the equation of the regression lines) corresponded to the moment at which half of the embryos were at the two-cell stage [28]. Uncleaved embryos after 32 hpa were fixed and stained with Hoechst 33342 to assess the nuclear status [6]. The percentage of cleaved embryos was defined as the number of cleaved embryos relative to total number of oocytes injected. Embryo stages then were recorded after 3 days of in vitro culture. After 7 days of culture, embryos were fixed and stained with Hoechst 33342 to assess blastomere numbers [6]. Embryos at the blastocyst stage were subjected to differential staining to determine the percentage of inner cell mass [27]. Experiment 1 and 2 values were expressed as mean ± standard deviation (SD) of the nine replicates. Percentage data were transformed using arcsine transformation before statistical analysis. Comparisons between treatments and among replicates were analyzed by ANOVA, Tukey multiple test for mean comparison, and Bartlett test for homogeneity of the variances. Data not normally distributed were analyzed by Kruskal-Wallis ANOVA on ranks and Dunn method for all pairwise comparisons. Differences were considered significant at P < 0.05 (SigmaStat).
RESULTS
Assessment of Sperm DNA Fragmentation and Presence of Centrosome in Sperm Suspensions Used for ICSI
In experiment 1, percentages of TUNEL-positive spermatozoa (Fig. 2, A and B) were not different (P > 0.05) in testicular (8.1 ± 1.5%) versus ejaculated (6.8 ± 2.2%) sperm suspensions used for ICSI. In both, testicular and ejaculated spermatozoa had comparable head, midpiece, and flagellar morphologies (Fig. 2, C and E). The head-midpiece junction of spermatozoon from both sources contained a pair of centrioles (Fig. 2, C and E). In experiment 2, sonication had no influence (P > 0.05) on percentages of TUNEL-positive sperm nuclei in testicular (7.8 ± 2.3%) versus ejaculated (7.6 ± 1.4%) sperm suspensions. The pair of centrioles remained attached to the midpiece after sonication regardless of the sperm source (Fig. 2, D and F).
FIG. 2.
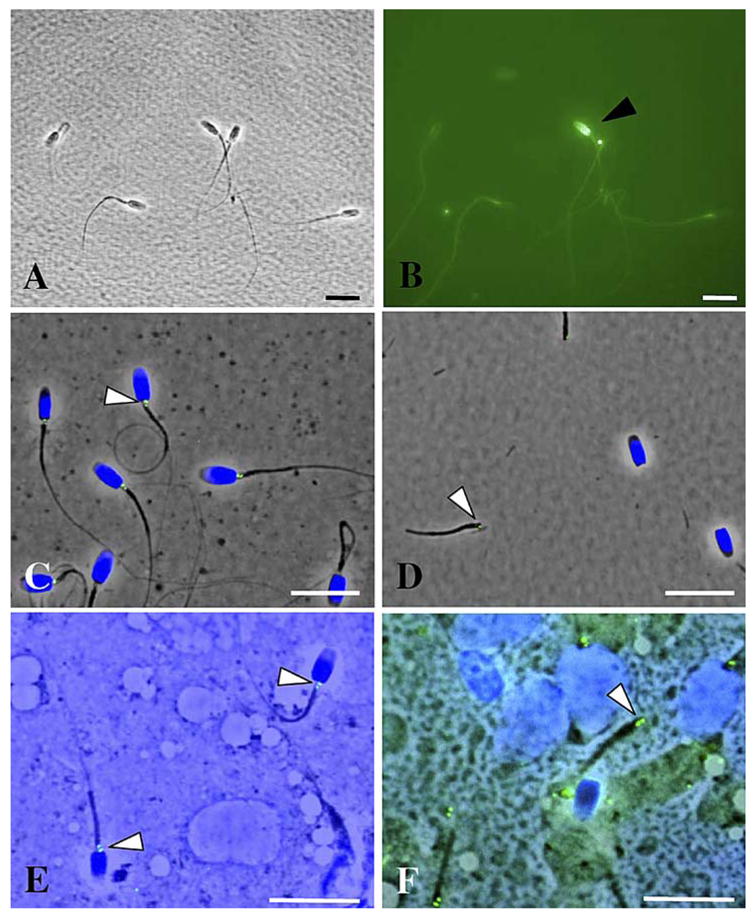
DNA integrity, morphology, and presence of centrosome in testicular and ejaculated spermatozoa used for ICSI. A) Ejaculated spermatozoa fixed for TUNEL assay (bright field). B) TUNEL-positive spermatozoon (black arrow) in the same ejaculated suspension (epifluorescence). C) Presence of centrosome (white arrow) revealed by immunostaining with anti-centrin antibodies in ejaculated spermatozoa. D) Attachment of centrosome (white arrow) to the midpiece after sonication of ejaculated sperm suspension. E) Presence of centro-some (white arrow) in testicular spermatozoa. F) Attachment of centrosome (white arrow) to the midpiece after sonication of testicular sperm suspension. Bar = 10 μm.
Evaluation of Centrosomal Function of Testicular and Ejaculated Spermatozoa
In experiment 1, percentages of fertilized versus cleaved oocytes after ICSI were not different within and between sperm sources (range, 62%–66%; P > 0.05). Unfertilized oocytes observed at 5 hpa or uncleaved oocytes fixed 32 hpa were still at the metaphase II stage regardless of the sperm source. Within those unfertilized oocytes, proportions of premature chromosome condensation (PCC) of the sperm nucleus (range, 35%–38%) and proportions of intact sperm heads (range, 62%–65%) were not different (P > 0.05) between ICSI with testicular versus ejaculated spermatozoa. At 5 hpa, fertilized oocytes always contained one male and one female pronucleus (identified by its proximity to the polar bodies; Fig. 3C). Male pronuclei had similar diameters (range, 9.6–10.2 μm; P > 0.05) regardless of the sperm source. Fertilized oocytes contained one of the three sperm aster types associated with the male pronucleus: 1) a large sperm aster with a defined focus of microtubules as well as elongations from the paternal chromatin towards the maternal chromatin (Fig. 3A), 2) a short sperm aster with a defined focus but no elongated microtubules (Fig. 3B), or 3) an absent sperm aster with no defined focus of microtubules and no extension toward the maternal chromatin (Fig. 3C). At 5 hpa, parthenogenetic controls from sham ICSI (~45% of oocytes activated) always revealed a single female pronucleus that was unable to organize an aster formation (Fig. 3D). Large sperm asters were the main configurations (P < 0.05) observed after ICSI with ejaculated spermatozoa and were never observed with testicular spermatozoa (Table 1). Short sperm asters were the main configurations (P < 0.05) after ICSI with testicular spermatozoa and their proportions were higher (P < 0.05) than with ejaculated spermatozoa (Table 1). Male pronuclei with absent sperm asters only were observed after ICSI with testicular spermatozoa (Table 1). Poor pattern of sperm aster formation then was associated with a delayed first cleavage (P < 0.05) after ICSI with testicular compared to ejaculated spermatozoa (Table 1). After 3 days of in vitro culture, embryos had equal-size blastomeres but fewer embryos (P < 0.05) had developed beyond the eight-cell stage after ICSI with testicular compared to ejaculated spermatozoa (Table 1). After 7 days of culture, percentages of embryos arrested at the 8–16-cell stage were higher (P < 0.05) after ICSI with testicular compared to ejaculated spermatozoa (Table 1). Conversely, percentages of morulae and blastocysts were higher (P < 0.05) at this time after ICSI with ejaculated spermatozoa (Table 1). Total blastomere numbers per blastocyst (range, 85–95) as well as percentages of inner cell mass (range, 20%–25%) were not influenced (P > 0.05) by sperm source.
FIG. 3.
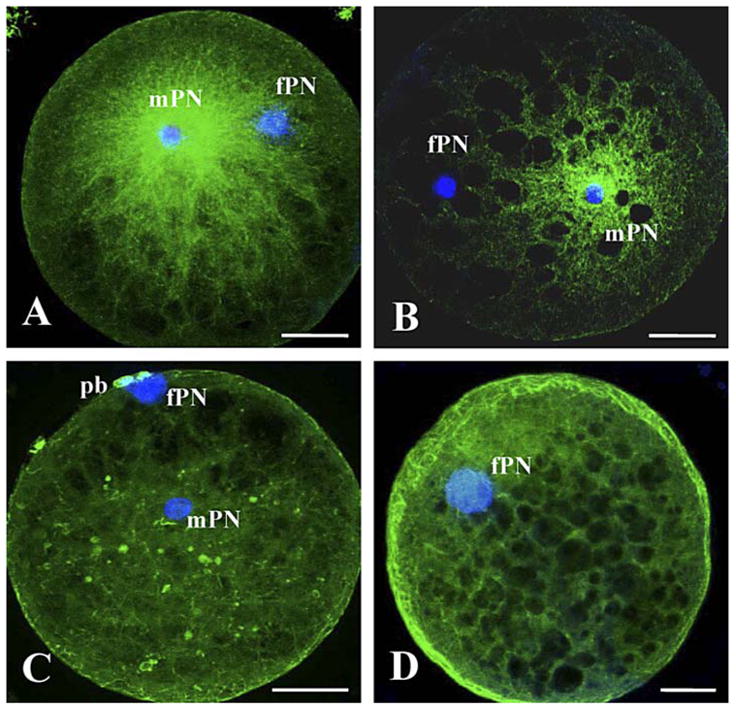
Sperm aster configurations (stained with FITC) in oocytes at 5 hpa after ICSI with intact testicular or ejaculated spermatozoa (A–C) and after sham ICSI (D). A) Large sperm aster. B) Short sperm aster. C) Absent sperm aster. mPN, Male pronucleus; fPN, female pronucleus; pb, polar body. Bar = 30 μm.
TABLE 1.
Pattern of sperm aster formation, timing of first cell cycle, and proportions of different embryo stages after ICSI with testicular or ejaculated sperm.*
| Sperm aster at 5 hpa (% of fertilized oocytes)
|
After 3 days of IVC (% of fertilized oocytes)
|
After 7 days of IVC (% of fertilized oocytes)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Large | Short | Absent | Time of first cleavage (hpa) | 2–8 Cells | >8 Cells | <8 Cells | 8–16 Cells | Morulae | Blastocysts | |
| Testicular sperm | 0a,A | 73.8 ± 8.0a,C | 26.2 ± 8.0a,B | 28.9 ± 1.0a | 55.0 ± 5.4a | 45.0 ± 5.4a | 20.0 ± 4.8a | 58.1 ± 4.8a | 9.5 ± 2.8a | 12.4 ± 3.8a |
| Ejaculated sperm | 73.2 ± 4.9b,A | 26.8 ± 4.9b,B | 0b,C | 24.1 ± 0.9b | 19.2 ± 4.6b | 80.8 ± 4.6b | 16.3 ± 2.8a | 34.6 ± 2.5b | 22.3 ± 3.4b | 26.8 ± 2.5b |
Values are expressed as means ± SD; hpa, hours postactivation; IVC, in vitro culture.
Within the same column, values with different lowercase superscripts differ significantly (P < 0.05).
Within the same row, sperm aster configurations with different uppercase superscripts differ significantly (P < 0.05).
Effect of Centrosome Source on Sperm Aster Organization and Embryo Development
Experiment 2 examined the impact of centrosome source on sperm aster formation, timing of the first cell cycle, and embryo development. Percentages of either fertilized versus cleaved oocytes were not different within and between the four combinations of sperm heads and centrosome/midpieces (range, 61%–65%; P > 0.05). Unfertilized oocytes observed at 5 hpa or uncleaved oocytes fixed 32 hpa were still at the metaphase II stage regardless of the ICSI treatment. Within those unfertilized oocytes, proportions of PCC of the sperm nucleus (range, 33%–37%) and proportions of intact sperm head (range, 63%–67%) were not different (P > 0.05) among ICSI with the different sperm head and centrosome/midpiece combinations. At 5 hpa, fertilized oocytes always contained one male and one female pronucleus (identified by its proximity to the polar bodies; Fig. 4, A, E, and G). At 5 hpa, male pronuclei had similar diameter (range, 9.8–10.1 μm; P > 0.05) regardless of the sperm head and centrosome/midpiece combination. As observed in experiment 1, fertilized oocytes contained one of the three different sperm aster configurations (large, Fig. 4, AC; short, Fig. 4, D–F; or absent, Fig. 4, G and H) with a sperm nucleus always proximal to the microtubule focus. Similar to experiment 1, parthenogenetic controls from sham ICSI (~45% of oocytes activated) proved that a single female pronucleus was unable to organize an aster formation (Fig. 4I). Large sperm asters were the main configurations (P < 0.05) observed in oocytes injected with a combination of ejaculated centro-some/midpiece along with a testicular or ejaculated sperm head (Table 2). However, no large sperm asters were observed in oocytes injected with a combination of testicular centrosome/ midpiece with a testicular or ejaculated sperm head (Table 2). Short sperm asters were the predominant configurations (P < 0.05) in oocytes injected with testicular centrosome/midpiece with either a testicular or an ejaculated sperm head (Table 2). There were higher proportions (P < 0.05) of short sperm asters in oocytes injected with testicular compared to ejaculated centrosome/midpiece combinations (Table 2). Male pronuclei with absent sperm asters were noted only in oocytes injected with testicular centrosome/midpiece combinations (Table 2). In the presence of poor sperm aster formation, there again was a delay in first cleavage (P < 0.05) when oocytes were injected with testicular centrosome/midpieces compared to injection with ejaculated centrosome/midpieces (Table 2). After 3 days of in vitro culture, embryos had equal-size blastomeres, but fewer embryos (P < 0.05) had developed beyond the eight-cell stage after ICSI with the testicular centrosome/midpiece combinations compared to those injected with centrosome/ midpieces from ejaculated spermatozoa (Table 2). This disadvantage was extended after 7 days of culture in that the use of combinations with testicular centrosome/midpiece combinations resulted in more embryos arrested at the 8–16-cell stage (P < 0.05) and fewer morulae and blastocysts (P < 0.05) than using centrosome/midpieces from ejaculated counterparts (Table 2). As in Experiment 1, there was no impact of ICSI treatment (P > 0.05) on total blastomere number per blastocyst (range, 83–96) or percentages of the inner cell mass (range, 18%–24%).
FIG. 4.
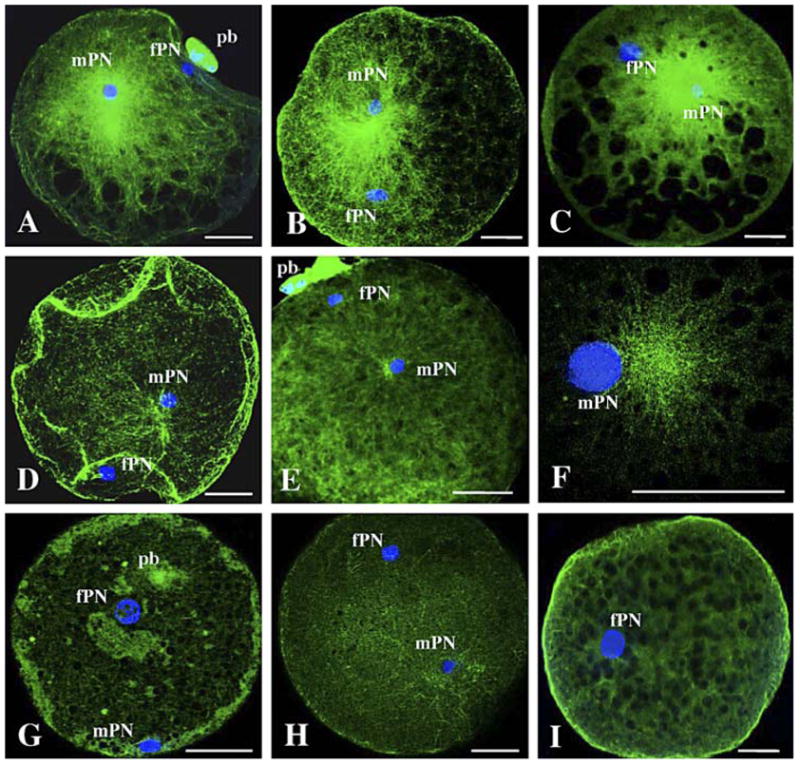
Sperm aster configurations (stained with FITC) in oocytes at 5 hpa after ICSI with sonicated testicular or ejaculated spermatozoa and centrosome/midpiece replacement (A–H) and after sham ICSI (I). AC) Large sperm asters. D and F) Short sperm asters. G and H) Absent sperm asters. mPN, Male pronucleus; fPN, female pronucleus; pb, polar bodies. Bar = 30 μm.
TABLE 2.
Pattern of sperm aster formation, timing of first cleavage, and proportions of embryo stages after ICSI with different combinations of sperm heads and centrosome/midpieces.*
| Sperm aster at 5 hpa (% of fertilized oocytes)
|
After 3 days of IVC (% of fertilized oocytes)
|
After 7 days of IVC (% of fertilized oocytes)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Combination† | Large | Short | Absent | Time of first cleavage (hpa) | 2–8 Cells | >8 Cells | <8 Cells | 8–16 Cells | Morulae | Blastocysts |
| Th + Tc | 0a,A | 79.6 ± 3.5a,C | 20.4 ± 3.5a,B | 29.5 ± 0.9a | 54.6 ± 4.6a | 45.4 ± 4.6a | 28.1 ± 4.1a | 57.1 ± 3.0a | 8.0 ± 5.5a | 6.8 ± 2.1a |
| Th + Ec | 71.7 ± 1.7b,A | 28.3 ± 1.7b,B | 0b,C | 24.4 ± 1.3b | 22.2 ± 6.4b | 77.8 ± 6.4b | 26.7 ± 2.8a | 31.6 ± 3.5b | 19.7 ± 3.0b | 22.0 ± 5.3b |
| Eh + Ec | 71.6 ± 5.9b,A | 28.4 ± 5.9b,B | 0b,C | 24.3 ± 1.2b | 23.2 ± 4.8b | 76.8 ± 4.8b | 20.3 ± 4.4a | 33.6 ± 5.2b | 23.0 ± 2.0b | 23.1 ± 4.7b |
| Eh + Tc | 0a,A | 78.6 ± 2.4a,C | 21.4 ± 2.4a,B | 29.2 ± 1.1a | 56.8 ± 6.6a | 43.2 ± 6.7a | 27.9 ± 4.5a | 52.7 ± 5.4a | 8.6 ± 4.5a | 10.8 ± 4.0a |
Values are expressed as means ± SD; hpa, hours postactivation; IVC, in vitro culture.
Sperm heads: Th, testicular; Eh, ejaculated; centrosome/midpieces: Tc, testicular; Ec, ejaculated.
Within the same column, values with different lowercase superscripts differ significantly (P < 0.05).
Within the same row, sperm aster configurations with different uppercase superscripts differ significantly (P < 0.05).
DISCUSSION
The present results clearly demonstrated the significant impact of the sperm proximal centriole and a need for its maturation to ensure appropriate timing of first cleavage and success of in vitro embryo development in the domestic cat. These findings helped clarify that compromised embryo development after oocyte injection with testicular spermatozoa was not a result of a higher incidence of DNA fragmentation from intragonadal spermatozoa. Rather, the immaturity of testicular spermatozoa appeared expressed by deficits in centrosomal functions, which gave rise to a poor aster formation that contributed to a delayed first cleavage, a slower developmental rate, and ultimately fewer morula and blastocyst formations. Further affirmation for this mechanism was provided by replacing the immature centrosome with a centrosome/midpiece from an ejaculated spermatozoon, an approach that enhanced embryo development. This is the first report in felids on the essential role of the proximal centriole in embryo development and emphasizes the importance of its maturation (most likely via epididymal transit) to ensure normal embryogenesis.
We originally hypothesized that reduced formation of morulae and blastocysts after ICSI with testicular spermatozoa could be due to a higher incidence of sperm DNA fragmentation. This was not the case because incidence of DNA fragmentation was identical between sperm suspensions used for ICSI and was not related to the differences in embryo developmental quality. Although the testicular and ejaculated cat spermatozoa used in these studies were subjected to different collection, storage, and selection techniques before ICSI, DNA fragmentation was similar in both sperm populations, with a similar incidence to that measured previously in normal human ejaculated spermatozoa [13]. It actually is in opposition with the hypothesis of defective sperm elimination during epididymal transit in the domestic cat [19] and requires further studies. As previously demonstrated in other species, sonication likely damaged membranes but did not impair sperm DNA integrity and the ability to induce normal embryo development [29, 30].
As observed previously, the sperm source (from within the gonad or ejaculate) had no impact on the proportion of fertilized oocytes, incidence of PCC, or the occurrence of intact sperm heads in unfertilized oocytes [6]. Testicular and ejaculated spermatozoa (with or without sonication) had the capacity to fertilize oocytes and formed comparably sized male pronuclei at 5 hpa, which indicated synchronized dynamics of oocyte activation and sperm chromatin decondensation [23]. A study in the rat [31] suggested that less condensed chromatin within immature sperm nuclei compromised fertilization in vitro. In contrast, our observations after centrosome/midpiece replacement affirmed that sperm head source (with less condensed chromatin in testicular sperm nucleus [18]) had no impact on cat oocyte fertilization and embryo development. In this respect, the cat was quite similar to the human, where different chromatin condensations in testicular versus ejaculated spermatozoa also have no influence on oocyte activation or quality of embryo development after ICSI [32].
For the cat, centrosomal function appeared to be a causative factor in compromised embryo development after ICSI with testicular spermatozoa. In turn, sperm aster morphology was highly reflective of the developmental potential. The association between presence of large sperm aster and better morula-blastocyst formation has been observed previously in bovine in vitro fertilization [23]. In that study, the presence of a large-diameter sperm aster was related to a 50% increase in advanced embryo formation. Pattern of sperm aster formation is considered to be under centrosomal control because no other element from the midpiece is involved in that process [33]. It was interesting that, in the absence of a sperm aster surrounding the male pronucleus, maternally derived microtu-bules (e.g., maternal γ-tubulin) could organize, take over the role of pronuclear movements, and complete a zygotic cycle (even if it was longer). However, the mechanism was likely different to that observed in unfertilized activated cat, rabbit, or porcine oocytes [6, 34, 35] because cell cycles are faster in parthenogenetic cat oocytes [6]. We determined that not all ejaculated cat spermatozoa had the capacity to form large sperm asters. About 25% of motile ejaculated cat spermatozoa produced short sperm asters. Furthermore, fertilized oocytes with large sperm asters did not always give rise to the formation of a morula or a blastocyst. Inversely, a few oocytes with short sperm aster (after ICSI with testicular spermatozoa) could develop to the morula-blastocyst stage. Clearly, the data revealed that, although sperm aster formation and male pronuclear formation were important checkpoints during the first embryonic cell cycle (as in other species [36, 37]), other paternal components (e.g., sperm nuclear theca [38]) were no doubt involved in early embryo developmental success in the domestic cat.
Our findings demonstrated, for the first time in the domestic cat, that first cell cycle kinetics was under paternal control (expressed here through the centrosome) and influenced subsequent embryo development like in human and cattle [39, 37]. The mechanism whereby the centrosome of an intragonadal cat spermatozoon adversely affects embryo development probably is related to the timing of pronuclear migration and apposition as well as mitotic spindle formation, as demonstrated in other species [23, 40, 41]. The delayed and slowed subsequent cell cycles related to poor microtubule organization eventually led to more developmental arrest at the 8–16-cell stage. Clearly, as in bovine [42] or porcine embryos [43], timing of cell cycles before the 8–16-cell stage was critical and impacted the subsequent embryo development. Embryonic genome activation, which occurs by the eight-cell stage in the cat [44], was likely compromised in those slow-cleaving embryos. However, other potential points of compromise from using spermatozoa with a dysfunctional centrosome deserve attention. For example, although blastomere size, blastomere number, and proportional inner cell mass were identical to that observed using ejaculated spermatozoa, the blastocysts produced with testicular centrosomes may be experiencing a higher incidence of aneuploidy [45, 46], an area of current study in our laboratory.
Our data revealed, for the first time, that the centrosome of testicular cat spermatozoa had a lower capacity to nucleate maternal microtubules that, in turn, altered first cell cycle kinetics without preventing cellular divisions. This centrosomal “immaturity” differs from centrosomal abnormalities reported in human and nonhuman primate testicular and ejaculated spermatozoa that lead to blocks at the pronuclear stage [25, 26, 47]. More specifically, centrosomal maturation has been described as the change in microtubule nucleation potential that occurs as cells in general pass through specific phases of the cell cycle [48]. In the case of paternal centrosomal maturation, sperm centrioles do not contain associated centrosomal proteins, which are mainly brought by the oocyte [41, 48]. The manner in which nucleation activity of sperm centrosomes influences microtubule length (by attracting γ-tubulin) is complex and not well understood [23, 33, 49, 50]. Number of microtubule nucleating sites in the testicular sperm centrosome may vary, or immature centrosome may nucleate microtubules that are more prone to detachment from the centrosome [23]. Given the clear difference between testicular and ejaculated spermatozoa, we suspect that full centrosomal maturation is likely acquired during epididymal transit since developmental potential between cat ejaculated and epididymal spermatozoa are comparable [3, 5, 6]. If occurring during epididymal transit, then centrosomal maturation most likely could be related to acquisition of sperm motility, new cytosolic proteins, or protein phosphorylations [17]. Other investigations are currently comparing centrosomal functions and presence of centriole maturation markers (e.g., cenexin, [51]) in sperm isolated from different regions of the epididymis.
Finally, our experimental design that allowed centrosomal replacement via ICSI into target oocytes had several benefits. Certainly, finding uncompromised embryo development after placing a centrosome from an ejaculated spermatozoon into an oocyte that also was the recipient of a testicular sperm head provided irrefutable evidence for the essential role of a mature proximal centriole. After ICSI with sonicated spermatozoa, sperm head and centrosome/midpieces remained sufficiently proximal to interact and allow pronuclear alignment as well as linear migration (like after ICSI or in vitro fertilization with an intact spermatozoon [52]). Likewise, the absence of arrest during the first cell cycle proved that the sperm head remained adjacent to the centrosome/midpiece [47]. Therefore, reorientation and elongation of microtubule array toward the female pronucleus, which represent good indicators of sperm aster quality [23, 53], were possible after centrosome replacement from an ejaculated sperm. This observation naturally suggested the potential value of this replacement strategy for therapeutic remediation in cases of ICSI using testicular gametes. This solution already has been proposed in other species with promising preliminary results [54, 55]. A related approach would also involve improving sperm aster formation, for example, via paclitaxel (a microtubule-stabilizing drug) exposure after ICSI [56]. In the case of animals, it also would be interesting to explore the benefits of cross-species centrosome replacement (e.g., using the centrosome from domestic cat ejaculated spermatozoa and a sperm head from wild felid species). Regardless of this technical feasibility, our collective findings illustrate fundamental aspects of felid testis biology that has never been observed in other mammals studied to date and the value of more studies of the centrosome as an essential critical functional unit of the spermatozoon.
Acknowledgments
We thank Drs. Michael Cranfield and Brent Whitaker (Maryland Line Animal Rescue) and Dr. Darby Thornburgh (Petworth Animal Hospital) for providing domestic cat ovaries and testes. The authors also thank Paul Leo and Amalia Dutra (National Human Genome Research Institute, National Institutes of Health) for assisting with confocal microscopy.
Footnotes
Supported by the Challinor Fellowship in Reproductive Sciences to P.C. from the Friends of the National Zoo and the National Institutes of Health (KO1 RR020045).
References
- 1.Honaramooz A, Snedaker A, Dobrinski I. A game of cat and mouse: xenografting of testis tissue from domestic kittens results in complete cat spermatogenesis in a mouse host. J Androl. 2004;25:926–930. doi: 10.1002/j.1939-4640.2004.tb03163.x. [DOI] [PubMed] [Google Scholar]
- 2.Pukazhenthi BS, Comizzoli P, Travis AJ, Wildt DE. Applications of emerging technologies to the study and conservation of threatened and endangered species. Reprod Fertil Dev. 2006;18:77–90. doi: 10.1071/rd05117. [DOI] [PubMed] [Google Scholar]
- 3.Pope CE, Johnson CA, McRae MA, Keller GL, Dresser BL. Development of embryos produced by intracytoplasmic sperm injection of cat oocytes. Anim Reprod Sci. 1998;53:221–236. doi: 10.1016/s0378-4320(98)00115-8. [DOI] [PubMed] [Google Scholar]
- 4.Gomez MC, Pope CE, Harris R, Davis A, Mikota S, Dresser BL. Births of kittens produced by intracytoplasmic sperm injection of domestic cat oocytes matured in vitro. Reprod Fertil Dev. 2000;12:423–433. doi: 10.1071/rd00126. [DOI] [PubMed] [Google Scholar]
- 5.Bogliolo L, Leoni G, Ledda S, Naitana S, Zedda M, Carluccio A, Pau S. Intracytoplasmic sperm injection of in vitro matured oocytes of domestic cats with frozen-thawed epididymal spermatozoa. Theriogenology. 2001;56:955–967. doi: 10.1016/s0093-691x(01)00621-5. [DOI] [PubMed] [Google Scholar]
- 6.Comizzoli P, Wildt DE, Pukazhenthi BS. In vitro development of domestic cat embryos following intra-cytoplasmic sperm injection with testicular spermatozoa. Theriogenology supplement. 2006 Feb 10; doi: 10.1016/j.theriogenology.2006.01.038. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 7.Yanagimachi R. Intracytoplasmic injection of spermatozoa and spermato-genic cells: its biology and applications in humans and animals. Reprod Biomed Online. 2005;10:247–288. doi: 10.1016/s1472-6483(10)60947-9. [DOI] [PubMed] [Google Scholar]
- 8.Van Steirteghem A, Nagy P, Joris H, Janssenswillen C, Staessen C, Verheyen G, Camus M, Tournaye H, Devroey P. Results of intracyto-plasmic sperm injection with ejaculated, fresh and frozen-thawed epididymal and testicular spermatozoa. Hum Reprod. 1998;13:134–142. doi: 10.1093/humrep/13.suppl_1.134. [DOI] [PubMed] [Google Scholar]
- 9.Hewitson L, Martinovich C, Simerly C, Takahashi D, Schatten G. Rhesus offspring produced by intracytoplasmic injection of testicular sperm and elongated spermatids. Fertil Steril. 2002;77:794–801. doi: 10.1016/s0015-0282(01)03281-2. [DOI] [PubMed] [Google Scholar]
- 10.Benchaib M, Braun V, Lornage J, Hadj S, Salle B, Lejeune H, Guerin JF. Sperm DNA fragmentation decreases the pregnancy rate in an assisted reproductive technique. Hum Reprod. 2003;18:1023–1028. doi: 10.1093/humrep/deg228. [DOI] [PubMed] [Google Scholar]
- 11.Larson-Cook KL, Brannian JD, Hansen KA, Kasperson KM, Aamold ET, Evenson DP. Relationship between the outcomes of assisted reproductive techniques and sperm DNA fragmentation as measured by the sperm chromatin structure assay. Fertil Steril. 2003;80:895–902. doi: 10.1016/s0015-0282(03)01116-6. [DOI] [PubMed] [Google Scholar]
- 12.Seli E, Gardner DK, Schoolcraft WB, Moffatt O, Sakkas D. Extent of nuclear DNA damage in ejaculated spermatozoa impacts on blastocyst development after in vitro fertilization. Fertil Steril. 2004;82:378–383. doi: 10.1016/j.fertnstert.2003.12.039. [DOI] [PubMed] [Google Scholar]
- 13.Tesarik J, Greco E, Mendoza C. Late, but not early, paternal effect on human embryo development is related to sperm DNA fragmentation. Hum Reprod. 2004;19:611–615. doi: 10.1093/humrep/deh127. [DOI] [PubMed] [Google Scholar]
- 14.Franca LR, Godinho CL. Testis morphometry, seminiferous epithelium cycle length, and daily sperm production in domestic cats (Felis catus) Biol Reprod. 2003;68:1554–1561. doi: 10.1095/biolreprod.102.010652. [DOI] [PubMed] [Google Scholar]
- 15.Manandhar G, Schatten H, Sutovsky P. Centrosome reduction during gametogenesis and its significance. Biol Reprod. 2005;72:2–13. doi: 10.1095/biolreprod.104.031245. [DOI] [PubMed] [Google Scholar]
- 16.Axner E, Linde-Forsberg C, Einarsson S. Morphology and motility of spermatozoa from different regions of the epididymal duct in the domestic cat. Theriogenology. 1999;52:767–778. doi: 10.1016/S0093-691X(99)00170-3. [DOI] [PubMed] [Google Scholar]
- 17.Turner TT. On the epididymis and its role in the development of the fertile ejaculate. J Androl. 1995;16:292–298. [PubMed] [Google Scholar]
- 18.Hingst O, Blottner S, Franz C. Chromatin condensation in cat spermatozoa during epididymal transit as studied by aniline blue and acridine orange staining. Andrologia. 1995;27:275–279. doi: 10.1111/j.1439-0272.1995.tb01105.x. [DOI] [PubMed] [Google Scholar]
- 19.Murakami M, Iwanaga S, Nishida T, Aiba T. Phagocytosis of latex beads by the epithelial cells in the terminal region of the vas deferens of the cat: SEM and TEM study. Andrologia. 1984;16:548–553. doi: 10.1111/j.1439-0272.1984.tb00411.x. [DOI] [PubMed] [Google Scholar]
- 20.Penfold LM, Jost L, Evenson DP, Wildt DE. Normospermic versus teratospermic domestic cat sperm chromatin integrity evaluated by flow cytometry and intracytoplasmic sperm injection. Biol Reprod. 2003;69:1730–1735. doi: 10.1095/biolreprod.103.016089. [DOI] [PubMed] [Google Scholar]
- 21.Schatten G. The centrosome and its mode of inheritance: the reduction of the centrosome during gametogenesis and its restoration during fertilization. Dev Biol. 1994;165:299–335. doi: 10.1006/dbio.1994.1256. [DOI] [PubMed] [Google Scholar]
- 22.Asch R, Simerly C, Ord T, Ord VA, Schatten G. The stages at which human fertilization arrests: microtubule and chromosome configurations in inseminated oocytes which failed to complete fertilization and development in humans. Hum Reprod. 1995;10:1897–1906. doi: 10.1093/oxfordjournals.humrep.a136204. [DOI] [PubMed] [Google Scholar]
- 23.Navara CS, First NL, Schatten G. Phenotypic variations among paternal centrosomes expressed within the zygote as disparate microtubule lengths and sperm aster organization: correlations between centrosome activity and developmental success. Proc Natl Acad Sci U S A. 1996;93:5384–5388. doi: 10.1073/pnas.93.11.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simerly C, Wu GJ, Zoran S, Ord T, Rawlins R, Jones J, Navara C, Gerrity M, Rinehart J, Binor Z. The paternal inheritance of the centrosome, the cell’s microtubule-organizing center, in humans, and the implications for infertility. Nat Med. 1995;1:47–52. doi: 10.1038/nm0195-47. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura S, Terada Y, Horiuchi T, Emuta C, Murakami T, Yaegashi N, Okamura K. Human sperm aster formation and pronuclear decondensation in bovine eggs following intracytoplasmic sperm injection using a Piezo-driven pipette: a novel assay for human sperm centrosomal function. Biol Reprod. 2001;65:1359–1363. doi: 10.1095/biolreprod65.5.1359. [DOI] [PubMed] [Google Scholar]
- 26.Rawe VY, Terada Y, Nakamura S, Chillik CF, Olmedo SB, Chemes HE. A pathology of the sperm centriole responsible for defective sperm aster formation, syngamy and cleavage. Hum Reprod. 2002;17:2344–2349. doi: 10.1093/humrep/17.9.2344. [DOI] [PubMed] [Google Scholar]
- 27.Comizzoli P, Wildt DE, Pukazhenthi BS. Effect of 1,2-propanediol versus 1,2-ethanediol on subsequent oocyte maturation, spindle integrity, fertilization, and embryo development in vitro in the domestic cat. Biol Reprod. 2004;71:598–604. doi: 10.1095/biolreprod.104.027920. [DOI] [PubMed] [Google Scholar]
- 28.Comizzoli P, Marquant-Le Guienne B, Heyman Y, Renard JP. Onset of the first S-phase is determined by a paternal effect during the G1-phase in bovine zygotes. Biol Reprod. 2000;62:1677–1684. doi: 10.1095/biolreprod62.6.1677. [DOI] [PubMed] [Google Scholar]
- 29.Kuretake S, Kimura Y, Hoshi K, Yanagimachi R. Fertilization and development of mouse oocytes injected with isolated sperm heads. Biol Reprod. 1996;55:789–795. doi: 10.1095/biolreprod55.4.789. [DOI] [PubMed] [Google Scholar]
- 30.Tateno H, Kimura Y, Yanagimachi R. Sonication per se is not as deleterious to sperm chromosomes as previously inferred. Biol Reprod. 2000;63:341–346. doi: 10.1095/biolreprod63.1.341. [DOI] [PubMed] [Google Scholar]
- 31.Chapman JC, Michael SD. Proposed mechanism for sperm chromatin condensation/decondensation in the male rat. Reprod Biol Endocrinol. 2003;1:20–27. doi: 10.1186/1477-7827-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hammadeh ME, al-Hasani S, Doerr S, Stieber M, Rosenbaum P, Schmidt W, Diedrich K. Comparison between chromatin condensation and morphology from testis biopsy extracted and ejaculated spermatozoa and their relationship to ICSI outcome. Hum Reprod. 1999;14:363–367. doi: 10.1093/humrep/14.2.363. [DOI] [PubMed] [Google Scholar]
- 33.Pinto-Correia C, Poccia DL, Chang T, Robl JM. Dephosphorylation of sperm midpiece antigens initiates aster formation in rabbit oocytes. Proc Natl Acad Sci U S A. 1994;91:7894–7898. doi: 10.1073/pnas.91.17.7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terada Y, Simerly CR, Hewitson L, Schatten G. Sperm aster formation and pronuclear decondensation during rabbit fertilization and development of a functional assay for human sperm. Biol Reprod. 2000;62:557–563. doi: 10.1095/biolreprod62.3.557. [DOI] [PubMed] [Google Scholar]
- 35.Kim BK, Lee YJ, Cui XS, Kim NH. Chromatin and microtubule organisation in maturing and pre-activated porcine oocytes following intracytoplasmic sperm injection. Zygote. 2002;10:123–129. doi: 10.1017/s0967199402002174. [DOI] [PubMed] [Google Scholar]
- 36.Ramalho-Santos J, Sutovsky P, Simerly C, Oko R, Wessel GM, Hewitson L, Schatten G. ICSI choreography: fate of sperm structures after monospermic rhesus ICSI and first cell cycle implications. Hum Reprod. 2000;15:2610–2620. doi: 10.1093/humrep/15.12.2610. [DOI] [PubMed] [Google Scholar]
- 37.Comizzoli P, Urner F, Sakkas D, Renard JP. Up-regulation of glucose metabolism during male pronucleus formation determines the early onset of the s phase in bovine zygotes. Biol Reprod. 2003;68:1934–1940. doi: 10.1095/biolreprod.102.011452. [DOI] [PubMed] [Google Scholar]
- 38.Sutovsky P, Schatten G. Paternal contributions to the mammalian zygote: fertilization after sperm-egg fusion. Int Rev Cytol. 2000;195:1–65. doi: 10.1016/s0074-7696(08)62703-5. [DOI] [PubMed] [Google Scholar]
- 39.Tesarik J, Mendoza C, Greco E. Paternal effects acting during the first cell cycle of human preimplantation development after ICSI. Hum Reprod. 2002;17:184–189. doi: 10.1093/humrep/17.1.184. [DOI] [PubMed] [Google Scholar]
- 40.Palermo GD, Colombero LT, Rosenwaks Z. The human sperm centrosome is responsible for normal syngamy and early embryonic development. Rev Reprod. 1997;2:19–27. doi: 10.1530/ror.0.0020019. [DOI] [PubMed] [Google Scholar]
- 41.Manandhar G, Schatten G. Centrosome reduction during Rhesus spermiogenesis: gamma-tubulin, centrin, and centriole degeneration. Mol Reprod Dev. 2000;56:502–511. doi: 10.1002/1098-2795(200008)56:4<502::AID-MRD8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 42.Lequarre AS, Marchandise J, Moreau B, Massip A, Donnay I. Cell cycle duration at the time of maternal zygotic transition for in vitro produced bovine embryos: effect of oxygen tension and transcription inhibition. Biol Reprod. 69:1707–1713. doi: 10.1095/biolreprod.103.017178. [DOI] [PubMed] [Google Scholar]
- 43.Schoenbeck RA, Peters MS, Rickords LF, Stumpf TT, Prather RS. Characterization of deoxyribonucleic acid synthesis and the transition from maternal to embryonic control in the 4-cell porcine embryo. Biol Reprod. 1992;47:1118–1125. doi: 10.1095/biolreprod47.6.1118. [DOI] [PubMed] [Google Scholar]
- 44.Hoffert KA, Anderson GB, Wildt DE, Roth TL. Transition from maternal to embryonic control of development in IVM/IVF domestic cat embryos. Mol Reprod Dev. 1997;48:208–215. doi: 10.1002/(SICI)1098-2795(199710)48:2<208::AID-MRD8>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 45.Silber S, Escudero T, Lenahan K, Abdelhadi I, Kilani Z, Munne S. Chromosomal abnormalities in embryos derived from testicular sperm extraction. Fertil Steril. 2003;79:30–38. doi: 10.1016/s0015-0282(02)04407-2. [DOI] [PubMed] [Google Scholar]
- 46.Hardarson T, Hanson C, Sjogren A, Lundin K. Human embryos with unevenly sized blastomeres have lower pregnancy and implantation rates: indications for aneuploidy and multinucleation. Hum Reprod. 2001;16:313–318. doi: 10.1093/humrep/16.2.313. [DOI] [PubMed] [Google Scholar]
- 47.Hewitson LC, Simerly CR, Tengowski MW, Sutovsky P, Navara CS, Haavisto AJ, Schatten G. Microtubule and chromatin configurations during rhesus intracytoplasmic sperm injection: successes and failures. Biol Reprod. 1996;55:271–280. doi: 10.1095/biolreprod55.2.271. [DOI] [PubMed] [Google Scholar]
- 48.Palazzo RE, Vogel JM, Schnackenberg BJ, Hull DR, Wu X. Centrosome maturation. Curr Top Dev Biol. 2000;49:449–470. doi: 10.1016/s0070-2153(99)49021-0. [DOI] [PubMed] [Google Scholar]
- 49.Sun QY, Lai L, Park KW, Kuhholzer B, Prather RS, Schatten H. Dynamic events are differently mediated by microfilaments, microtubules, and mitogen-activated protein kinase during porcine oocyte maturation and fertilization in vitro. Biol Reprod. 2001;64:879–889. doi: 10.1095/biolreprod64.3.879. [DOI] [PubMed] [Google Scholar]
- 50.Simerly C, Zoran SS, Payne C, Dominko T, Sutovsky P, Navara CS, Salisbury JL, Schatten G. Biparental inheritance of gamma-tubulin during human fertilization: molecular reconstitution of functional zygotic centrosomes in inseminated human oocytes and in cell-free extracts nucleated by human sperm. Mol Biol Cell. 1999;10:2955–2969. doi: 10.1091/mbc.10.9.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lange BM, Gull K. A molecular marker for centriole maturation in the mammalian cell cycle. J Cell Biol. 1995;130:919–927. doi: 10.1083/jcb.130.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Blerkom J, Davis P, Merriam J, Sinclair J. Nuclear and cytoplasmic dynamics of sperm penetration, pronuclear formation and microtubule organization during fertilization and early preimplantation development in the human. Hum Reprod Update. 1995;1:429–461. doi: 10.1093/humupd/1.5.429. [DOI] [PubMed] [Google Scholar]
- 53.Van Blerkom J, Davis P. Evolution of the sperm aster after microinjection of isolated human sperm centrosomes into meiotically mature human oocytes. Hum Reprod. 1995;10:2179–2182. doi: 10.1093/oxfordjournals.humrep.a136264. [DOI] [PubMed] [Google Scholar]
- 54.Moomjy M, Colombero LT, Veeck LL, Rosenwaks Z, Palermo GD. Sperm integrity is critical for normal mitotic division and early embryonic development. Mol Hum Reprod. 1999;5:836–844. doi: 10.1093/molehr/5.9.836. [DOI] [PubMed] [Google Scholar]
- 55.Emery BR, Thorp C, Malo JW, Carrell DT. Pregnancy from intracyto-plasmic sperm injection of a sperm head and detached tail. Fertil Steril. 2004;81:686–688. doi: 10.1016/j.fertnstert.2003.07.025. [DOI] [PubMed] [Google Scholar]
- 56.Nakamura S, Terada Y, Rawe VY, Uehara S, Morito Y, Yoshimoto T, Tachibana M, Murakami T, Yaegashi N, Okamura K. A trial to restore defective human sperm centrosomal function. Hum Reprod. 2005;20:1933–1937. doi: 10.1093/humrep/deh899. [DOI] [PubMed] [Google Scholar]


