Abstract
Fragile X syndrome (FXS), a common inherited form of mental retardation, is caused by the functional absence of the fragile X mental retardation protein (FMRP), an RNA-binding protein that regulates the translation of specific mRNAs at synapses. Altered synaptic plasticity has been described in a mouse FXS model. However, the mechanism by which the loss of FMRP alters synaptic function, and subsequently causes the mental impairment, is unknown. Here, in cultured hippocampal neurons, we used siRNAs against Fmr1 to demonstrate that a reduction of FMRP in dendrites leads to an increase in internalization of the α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (AMPAR) subunit, GluR1, in dendrites. This abnormal AMPAR trafficking was caused by spontaneous action potential-driven network activity without synaptic stimulation by an exogenous agonist and was rescued by 2-methyl-6-phenylethynyl-pyridine (MPEP), an mGluR5-specific inverse agonist. Because AMPAR internalization depends on local protein synthesis after mGluR5 stimulation, FMRP, a negative regulator of translation, may be viewed as a counterbalancing signal, wherein the absence of FMRP leads to an apparent excess of mGluR5 signaling in dendrites. Because AMPAR trafficking is a driving process for synaptic plasticity underlying learning and memory, our data suggest that hypersensitive AMPAR internalization in response to excess mGluR signaling may represent a principal cellular defect in FXS, which may be corrected by using mGluR antagonists.
Keywords: fragile X syndrome, autism
Persistent changes in synaptic efficacy are caused by signals that affect the trafficking and abundance of α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors (AMPARs) at synapses and have been proposed as a synaptic mechanism of learning and memory (1–4). Long-term potentiation can be expressed by AMPAR trafficking and insertion into the plasma membrane at synapses, which result in an increase of surface AMPARs (1–4). Conversely, long-term depression (LTD) is accompanied by regulated internalization and consequent loss of surface AMPARs (1–4). Changes in AMPAR composition have been reported in cognitive disorders such as Alzheimer's disease (5), schizophrenia (6), and fragile X syndrome (FXS) (7) and, therefore, have been implicated as a principal cellular mechanism for cognitive impairment. However, it is unknown whether the altered levels of AMPARs reported in these disorders can be attributed to imbalances in their regulated trafficking to and from synapses, which is a principal mechanism of synaptic plasticity.
FXS, the most common form of inherited mental retardation, is caused by the absence of fragile X mental retardation protein (FMRP), an mRNA-binding protein (8–11) that is present at dendritic spines (12) and appears to regulate local protein synthesis important for synaptic plasticity (9, 10). Dendritic spine defects have been described in both FXS patients and mouse Fmr1 knockout (KO) models (8–11). Presumably, the loss of translational regulation at dendritic spines underlies the cognitive impairment in FXS (9, 13). Because dendritic protein synthesis is required for some types of synaptic plasticity (3, 13), deficiency of a key translational regulator such as FMRP may lead to impaired synaptic plasticity. Indeed, in Fmr1 KO mice, group I mGluR-dependent LTD (mGluR-LTD), which requires protein synthesis in wild-type mice, is enhanced in hippocampal Schaffer collateral synapses of the CA1 area (14, 15) and in the cerebellar parallel fiber to Purkinje cell synapses (16). At wild-type synapses, with chemical or electrical stimulation to induce mGluR-LTD, persistent internalization of AMPAR occurs (1, 17, 18). Thus, a reasonable prediction based on the exaggerated LTD in Fmr1 KO mice is enhanced AMPAR internalization, although altered AMPAR trafficking has not been demonstrated in FXS models. Moreover, because the basal level of synaptic transmission by AMPAR in Fmr1 KO mice is comparable to wild-type mice (14), the mechanism by which (RS)-3,5-dihydroxyphenylglycine (DHPG) stimulation induces exaggerated LTD in Fmr1 KO mice is not clear. Here we show that there is indeed aberrant AMPAR trafficking in FMRP-deficient dendrites at the basal state without affecting the total amount of surface AMPAR and that this results from excessive mGluR5 signaling.
Results
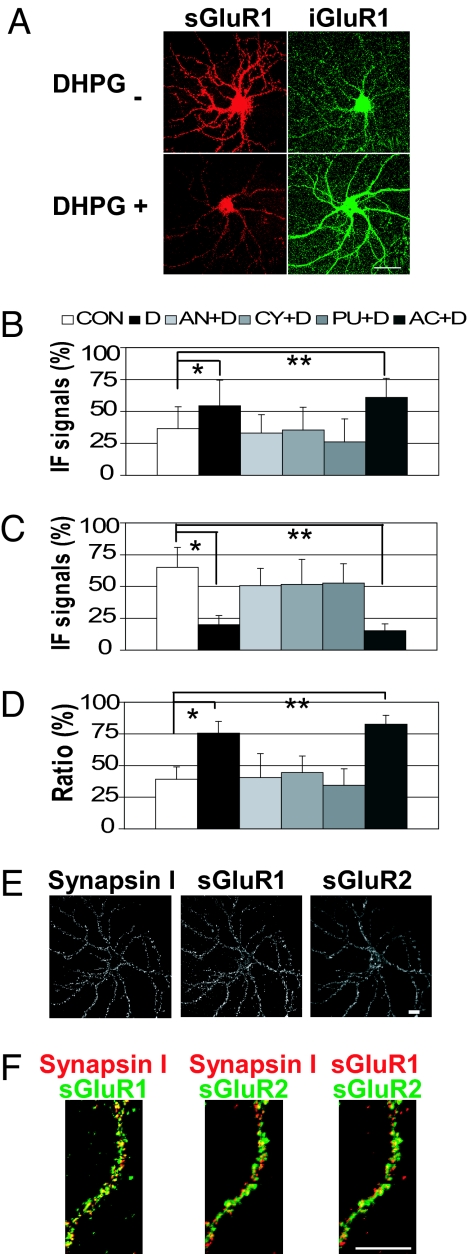
To test the hypothesis that altered levels of AMPAR internalization are an underlying molecular impairment of FMRP deficiency, we used a well characterized dual-staining method to assess surface receptor trafficking in cultured hippocampal neurons (19–21). The major advantage of this approach is that the dynamic trafficking of AMPAR can be visualized and quantified. To validate the assay, mGluR-dependent internalization of AMPARs in wild-type primary rat hippocampal neurons was first examined and quantified by digital image analysis. We detected basal levels of GluR1 internalization in unstimulated wild-type neurons (22). As expected from previous reports using other staining methods (17, 18), stimulation of neurons with DHPG, a group I mGluR-specific agonist that is known to induce mGluR-dependent LTD in the hippocampus (13), induced a clear reduction of surface-labeled GluR1s (≈71% in secondary dendrites) and a corresponding increase in internalized GluR1s (Fig. 1 A–D).
Fig. 1.
Staining of AMPARs in wild-type live neurons. (A) Representative images of DHPG-induced GluR1 internalization. Remaining surface and internalized GluR1 signals are labeled as sGluR1 and iGluR1, respectively. (Scale bar: 50 μm.) (B–D) Translation inhibitors block DHPG-induced internalization of GluR1. Mean IF signals of internalized GluR1 (B), labeled GluR1 remaining on the surface (C), and ratio of internalized GluR1 in total labeled GluR1 (i/t GluR1) in wild-type dendrites (D) (n = 15 per column). Error bars represent standard deviations. CON, control; D, DHPG, AN, anisomycin; CY, cycloheximide; PU, puromycin; AC, actinomycin D. (B) *, P = 1.3 × 10−2, **, P = 2.8 × 10−4. (C) *, P = 6.8 × 10−11; **, P = 2.7 × 10−12. (D) *, P = 4.4 × 10−11; **, P = 3.9 × 10−14. (E and F) Colocalization of surface GluR1, GluR2, and Synapsin I, a synaptic marker. (Scale bars: 20 μm.) (E) Representative IF images of a wild-type neuron stained for surface GluR1 (sGluR1) and surface GluR2 (sGluR2) under a nonpermeabilized condition followed by Synapsin I staining. (F) Higher magnification images of a dendrite showing colocalization of Synapsin I, sGluR1, and sGluR2 IF signals.
A previous report showed that inhibition of DHPG-induced loss of surface GluR1 with cycloheximide (added 15 min before DHPG administration) was detected at 60 min but not at 15 min after DHPG stimulation and led to a conclusion that protein synthesis is required only for determining the fate of internalized receptors, but not for their initial internalization (18). However, because DHPG-induced mGluR-LTD is translation-dependent throughout early and late phases (13), we next examined whether this GluR1 internalization induced by DHPG is translation-dependent. Neurons were preincubated with translation inhibitors of three distinct inhibition mechanisms before DHPG stimulation. Each inhibitor tested (anisomycin, puromycin, or cycloheximide) was effective in blocking the DHPG-induced internalization of GluR1 [Fig. 1 B–D and supporting information (SI) Fig. 5]. We determined that preincubation with cycloheximide for 45 min before DHPG administration blocks receptor internalization immediately after DHPG stimulation, as did as anisomycin and puromycin. In contrast, preincubation with a transcription inhibitor, actinomycin D, did not affect the DHPG-induced GluR1 internalization (Fig. 1 B–D and SI Fig. 5). Thus, our findings demonstrate a novel role for protein synthesis in the early phase of internalization of GluR1 in response to mGluR activation. These data verified that this staining method is able to detect translation-dependent trafficking of GluR1 in live neurons. Surface GluR1 or GluR2, as stained with this method under nonpermeabilized condition, was significantly colocalized with a synaptic marker, Synapsin I-positive puncta (Fig. 1 E and F), thereby demonstrating the specificity of this surface-labeling procedure to stain synaptic AMPARs in live neurons.
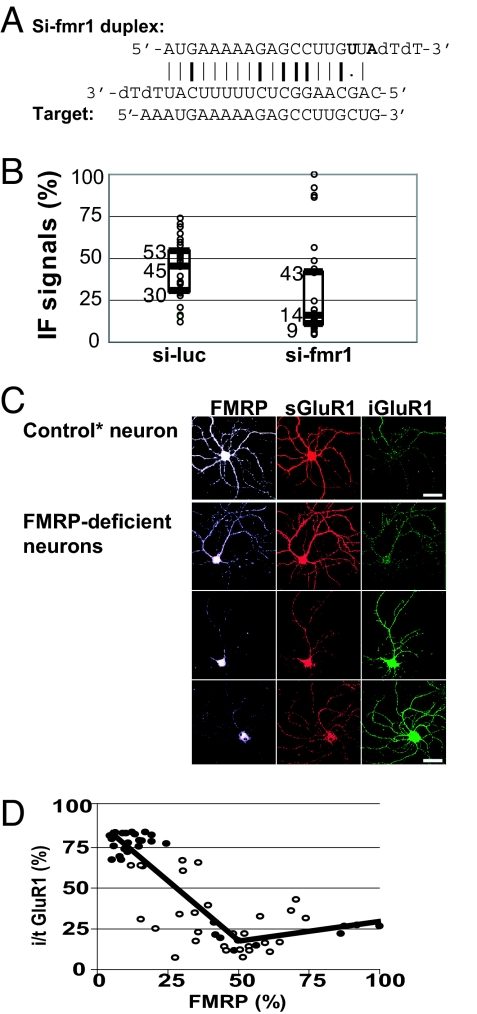
Next, to determine the effect of reduced FMRP on AMPAR trafficking, FMRP expression levels were knocked down in the neurons. We designed an siRNA, si-fmr1, specific to the Fmr1 sequence that does not share any homology to other known genes, including the paralogs Fxr1 and Fxr2 (Fig. 2A). This siRNA included two nucleotide changes to reduce stability at the 5′ end of the antisense strand because it normally facilitates the delivery of that strand to the RNA-induced silencing complex and knocks down a target more effectively (23). Using this approach of siRNA knockdown, as opposed to using cultured neurons from Fmr1 KO mice, allows measurement of the effects of a full gradient of FMRP expression in a population of cells present in a single culture prepared from a single animal. Immunocytochemistry with a monoclonal anti-FMRP antibody verified the marked reduction of FMRP in the dendrites of a majority of neurons transfected with si-fmr1, whereas FMRP levels in cell bodies also were decreased but not lost. The FMRP immunofluorescence (IF) signal was substantially diminished to a background level in ≈70% of the dendrites by day 4 after transfection with si-fmr1 as analyzed by quantitative digital image analysis (Fig. 2B). In contrast, neurons transfected with control siRNA (si-luc) showed modest variation of FMRP IF signals in the dendrites, compared with untransfected neurons (data not shown).
Fig. 2.
Reduction of FMRP by siRNA in dendrites correlates with a reduction of labeled GluR1 remaining on the surface and an increase of internalized GluR1. (A) Sequence of the siRNA duplex designed to target Fmr1. Induced mutations are shown in bold letters. G:C and A:U bonds and G:U wobble are indicated by bold lines, plain lines, and a dot, respectively. (B) Box plot of FMRP IF signals in individual dendrites (n = 30) transfected with si-luc or si-fmr1. Median, first quartile, and third quartile are indicated by middle, lower, and upper lines of the boxes and by numbers next to the lines. (C) IF images of representative neurons transfected with si-fmr1. Control neuron was chosen from si-fmr1-treated neurons that showed FMRP levels comparable to si-luc-treated control neurons. (Scale bars: 50 μm.) (D) Piecewise linear regression between FMRP and i/t GluR1 in si-luc- (open circles) and si-fmr1- (filled circles) transfected dendrites (n = 60). Calculated breakpoint is x = 49. Correlation P values are <0.05 if FMRP (%) is below the breakpoint.
Using these approaches, we simultaneously stained FMRP, internalized GluR1, and labeled GluR1 remaining on the surface in unstimulated si-luc- or si-fmr1-treated dendrites. Surprisingly, reductions in FMRP seemed to correlate, on a single dendrite level, with increases in internalized GluR1 IF signals and reductions in surface-remaining GluR1 at a basal state in unstimulated dendrites (Fig. 2C). To further evaluate the correlation of FMRP and GluR1 internalization, IF signals were quantified in individual dendrites. Total labeled GluR1 was determined by combining internalized GluR1 and remaining surface-labeled GluR1. Percentages of internalized GluR1 in total labeled GluR1 (i/t GluR1) in dendrites both in si-luc- and in si-fmr1-transfected neurons were plotted together and subjected to piecewise regression analyses, which demonstrated a significant negative correlation between FMRP and i/t GluR1 if FMRP levels were reduced to <49% of the maximum (Fig. 2D). We found a similar correlation of reduced FMRP levels with GluR2 internalization (SI Fig. 6). Thus, without an exogenously applied agonist, FMRP deficiency directly correlates with AMPAR internalization. These results provide the first link between altered AMPAR trafficking and FXS, a specific form of cognitive deficiency caused by the loss of FMRP.
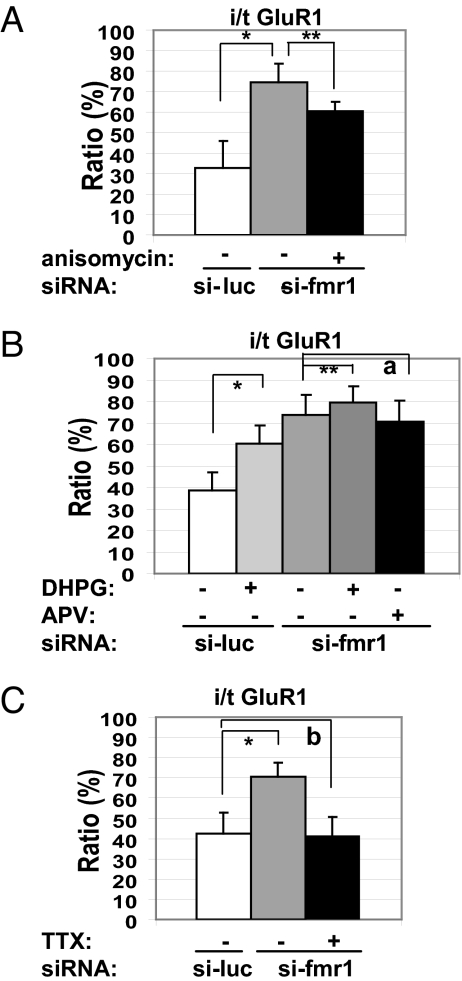
Characteristic features of mGluR-LTD reported in Fmr1 KO hippocampi are that it is not blocked by translation inhibitors (24) and it is enhanced compared with that in wild-type hippocampi (14). To determine whether the aberrant GluR1 internalization in FMRP-deficient dendrites shares characteristics with the electrophysiology, we applied a translation inhibitor under the same conditions that completely blocked DHPG-induced GluR1 internalization in wild-type dendrites (Fig. 1). We detected that the aberrant GluR1 internalization in FMRP-deficient dendrites was only partially blocked (Fig. 3A). In addition, exogenously applied DHPG induced additional GluR1 internalization in the FMRP-deficient dendrites, although it was less robust than that observed in control neurons (Fig. 3B).
Fig. 3.
Characterization of aberrant GluR1 internalization in FMRP-deficient dendrites (n = 21 per column). (A) A translation inhibitor partially rescued the aberrant GluR1 internalization. *, P = 1.07 × 10−14; **, P = 8.66 × 10−8. (B) DHPG-induced additional internalization. *, P = 3.14 × 10−14; **, P = 4.88 × 10−2 (a = 0.293). (C) Tetrodotoxin rescued the aberrant GluR1 internalization. *, P = 6.81 × 10−12 (b = 0.422).
This abnormal AMPAR trafficking occurred in FMRP-deficient dendrites in the absence of an externally applied agonist and may have resulted from spontaneous glutamatergic activation by either quantal release of glutamate or action potential-driven network activity, which do not normally induce AMPAR trafficking in wild-type neurons. Alternatively, the mGluR may be excitable in the absence of glutamate in FMRP-deficient neurons. To dissect the source of glutamate responsible for the aberrant GluR1 internalization, we blocked action potentials with tetrodotoxin and detected complete rescue in the FMRP-deficient dendrites (Fig. 3C).
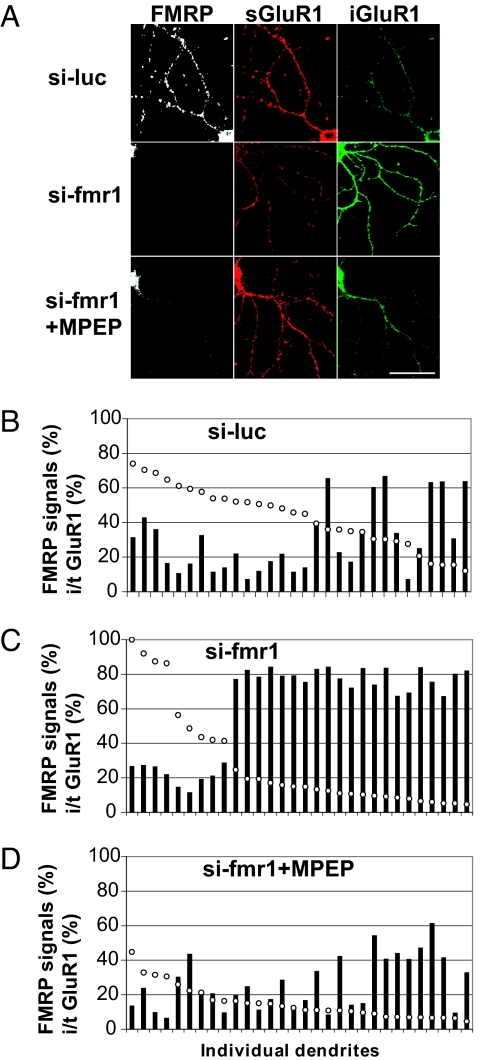
Next, we used glutamate receptor antagonists to identify the glutamatergic pathway responsible for the aberrant trafficking of GluR1 in unstimulated FMRP-deficient dendrites. Because the enhanced mGluR-LTD in Fmr1 KO mice has been attributed to enhanced group I mGluR signaling, especially mGluR5 (10), we reasoned that the mGluR5 signaling might be in excess in unstimulated FMRP-deficient dendrites. We applied 2-methyl-6-phenylethynyl-pyridine (MPEP), an mGluR5-specific inverse agonist, which can inhibit not only the agonist-induced activation of mGluR5, but also its constitutive activity (25), to Fmr1-knockdown neurons for 3 days before GluR1 IF evaluation at a concentration that does not affect NMDA receptors. Incubation of the cultured neurons with MPEP led to a significant retention of labeled GluR1 on the surface (Fig. 4A). Quantification of IF signal intensities of GluR1 and FMRP in individual dendrites further demonstrated that MPEP rescued the abnormal GluR1 internalization in FMRP-deficient dendrites (Fig. 4 B–D). Because the expression level of FMRP varied between dendrites, as expected (Fig. 4 B and C), we used digital image analysis to quantify GluR1 signals in si-fmr1-transfected neurons with the lowest FMRP levels (Fig. 4C). The same analysis was performed on an equal number of dendrites from si-luc-transfected neurons (Fig. 4B) and si-fmr1-transfected, MPEP-treated neurons (Fig. 4D). As in the previous analysis, the dendrites were sorted by FMRP levels to determine which should be used in the analysis. In Fmr1 knockdown dendrites, the internalized GluR1 was increased (18.2 ± 22.6% in si-luc-transfected dendrites to 42.2 ± 23.2%) (SI Fig. 7) compared with si-luc treated-cells, and the remaining surface-labeled GluR1 was reduced (27.8 ± 13.0% to 15.1 ± 5.7%), thus generating an increase in i/t GluR1 (33.5 ± 21.6% to 78.2 ± 5.5%), compared with control dendrites transfected with si-luc (SI Fig. 7). MPEP treatment rescued the changes in GluR1 localization (internalized, 10.3 ± 6.9%; surface labeled, 33.7 ± 12.8%), giving an i/t GluR1 comparable to the control dendrites (29.5 ± 15.9%) (SI Fig. 7). The lowest doses of MPEP to generate a complete recovery of i/t GluR1 in Fmr1 knockdown dendrites were 10 μM for 3 days or 20 μM for 16 h; the effects were the same with doses ≤50 μM. In contrast, LY367385, an mGluR1-specific antagonist, had only a modest effect of rescue (data not shown). These data demonstrate that the aberrant internalization of AMPARs in FMRP-deficient dendrites, caused by spontaneous, action potential-driven network activity, results from excessive mGluR5 signaling and that mGluR5 inverse agonists rescue these fundamental consequences of the absence of FMRP.
Fig. 4.
MPEP rescues the aberrant internalization of GluR1 after loss of FMRP. (A) Representative IF images of transfected dendrites. (Scale bar: 50 μm.) (B–D) Quantification of IF signals in individual dendrites. Open circles, FMRP IF signals; filled bars, i/t GluR1.
Discussion
It is well accepted that a driving process for the long-term synaptic plasticity underlying learning and memory involves the dynamic regulation of AMPAR trafficking in and out of the plasma membrane at glutamatergic synapses (1, 4). We discovered that the dynamic process of AMPAR internalization is negatively regulated by a specific translational regulator, FMRP. FMRP-deficient neurons were characterized by increased levels of internalized GluR1 (33.5–78.2%) as analyzed by using quantitative digital imaging analysis (SI Fig. 7). Moreover, our analysis of individual dendrites in siRNA-treated cultures revealed that i/t GluR1 correlated with the extent to which FMRP was reduced in a particular dendrite (Fig. 4 C and D). The pathological process in FXS has been hypothesized as the mGluR theory of FXS, wherein FMRP is assumed to function as a negative regulator of the mGluR signaling pathway, which normally regulates the local protein synthesis that is crucial for mGluR-LTD and, perhaps, activity-dependent internalization of AMPARs (10). Our data demonstrate that excessive AMPAR internalization is a direct consequence of FMRP deficiency and provide clear mechanistic evidence in support of this theory, which stems from findings that mGluR-LTD is enhanced in hippocampal slices from Fmr1 KO mice (14). The aberrant AMPAR trafficking in FMRP-deficient dendrites was predominantly translation-independent (SI Fig. 7) and reactive to DHPG, which was apparently occluded because of the exaggerated basal GluR1 internalization (Fig. 3B). A previous report has shown that bicuculline-induced prolonged epileptiform discharges occur independent of a group I mGluR agonist in Fmr1 KO mice (26). The increased internalization of AMPAR in FMRP-deficient dendrites at a basal state, reported here, is consistent with this observation and, indeed, suggests that excessive mGluR signaling may underlie the electrophysiological abnormalities in FXS. Thus, altered synaptic plasticity due to persistent AMPAR internalization may be a significant contributor to the well characterized cognitive deficit in FXS.
We observed no statistically significant change in total-labeled GluR1, which corresponds to the amount of surface-labeled and internalized GluR1, in FMRP-deficient dendrites (SI Fig. 7D). This result is in agreement with a previous report that demonstrated no change in membranous GluR1 at hippocampal synapses in Fmr1 KO mice by immunohistochemistry (7) and is consistent with an electrophysiological observation showing no difference in basal synaptic transmission in Fmr1 KO hippocampal slices (14). However, our results are in apparent conflict with a recent report that demonstrated increased surface GluR1 in hippocampal dendrites in cocultured Fmr1 KO neurons with wild-type neurons by using a different method of staining and quantification (27). Because our method stains surface-localized GluR1 at the point of antibody labeling in live neurons, newly inserted GluR1 to the membrane after the labeling would not be detectable. To evaluate possible steady-state differences in surface GluR1, we conducted surface GluR1 staining in fixed nonpermeabilized neurons transfected with si-fmr1 and found no statistical correlation of FMRP level and surface-labeled GluR1 (data not shown).
One recent study showed that postsynaptic expression of FMRP reduced the number of functional synapses (27). Of interest, FMRP mutants with impaired translation regulation were unable to modify these synaptic properties (27). Together with a report showing reduced association between mGluR5 and Homer protein, which anchors mGluR5 to PSD, in Fmr1 KO mice (28), it is likely that postsynaptic loss of FMRP-regulated translation leads to exaggerated mGluR5 signaling in dendrites and subsequent defects in AMPAR internalization.
We observed that ≈10% of dendrites showed paradoxically higher FMRP levels in neurons transfected with si-fmr1, compared with neurons transfected with control siRNA (Figs. 2B and 4C). Because it was not seen in neurons transfected with si-fmr1 and treated with MPEP (Fig. 4D), involvement of mGluR5 signaling is suggested. It has been reported that DHPG induces up-regulation of FMRP (29) and trafficking of FMRP to dendrites (30) in wild-type neurons. Therefore, we assume that enhanced mGluR5 signaling in the loss of FMRP in dendrites causes up-regulation and/or trafficking of FMRP to dendrites from cell body, in which FMRP level remains conserved, resulting in apparently higher FMRP levels in dendrites in a few neurons in which knockdown is relatively inefficient.
One facet of the mGluR theory is that mGluR antagonists should moderate the synaptic activity-induced excessive signaling that follows FMRP loss. In fact, compelling evidence for this notion has been reported. MPEP rescues the bicuculline-induced prolonged epileptiform discharges (26), as well as audiogenic seizures and open field activity defects (31) in Fmr1 KO mice. Additionally, in the Drosophila model lacking dfmr1, the ortholog of FMR1, drugs that inhibit mGluR signaling, including MPEP, rescue the behavioral phenotypes and abnormal brain structure (32). Most recently, MPEP also rescued zebrafish phenotypes induced by Fmr1 knockdown (33). Collectively, these data support the involvement of FMRP in the repression of mGluR signaling and suggest the use of mGluR inverse agonists as a possible therapeutic strategy for FXS. Our study added the importance of antagonizing the mGluR5 signaling pathway in FXS by demonstrating that the defect in FMRP-deficient dendrites is specific to the mGluR5 signaling and does not involve NMDAR-driven AMPAR internalization because APV, an NMDAR antagonist, did not rescue the phenomena (Fig. 3B). Furthermore, a recent report has shown that, in wild-type hippocampal neurons, mGluR1, but not mGluR5, has a predominant role in DHPG-induced mGluR-LTD and associated reduction of AMPAR from the surface (34). It is important to emphasize that our data demonstrated that, in FMRP-deficient neurons, the aberrant trafficking of AMPAR was only modestly blocked by an mGluR1-specific antagonist, but was completely rescued by MPEP. Together these data suggest that the pathological mechanism of aberrant AMPAR trafficking in FMRP deficiency is distinct from physiological DHPG-induced AMPAR trafficking and mGluR-LTD, suggesting that selective mGluR5 blockade could correct abnormal phenotype while preserving physiological mGluR1-dependent synaptic plasticity in FXS. In light of this finding, our observation of the ability of an mGluR5 inverse agonist to normalize the aberrant AMPAR trafficking in mammalian neurons provides substantial motivation for future therapeutic intervention for FXS.
Materials and Methods
Hippocampal Culture.
Primary hippocampal cultures were prepared from E18 rats as described (35) in accordance with the Institutional Animal Care and Use Committee guidelines. Cells were plated (2,000 cells per cm2) on poly(l-lysine)-coated Bioptechs coverslips (0.2 mg/ml) in MEM with FBS (10%) for 2 h, inverted onto dishes containing astroglia, and maintained in neurobasal media supplemented with B-27 and Gluta MAX-1 (Invitrogen, San Diego, CA).
Preparation of siRNA and Transfection.
siRNA duplexes were synthesized by using a Silencer siRNA Construction Kit (Ambion, Austin, TX). The sequences of the si-luc duplex are as follows: 5′-CGUACGCGGAAUACUUCGAdTdT-3′ and 5′-UCGAAGUAUUCCGCGUACGdTdT-3′. Si-fmr1 sequences are shown in Fig. 2. Primary neurons were transfected with the siRNA duplex at 14–18 DIV by using the calcium phosphate method (36) at the final concentration of 50 μM and were incubated for 4 additional days.
IF and Drug Treatment.
Tetrodotoxin (2 μM, 24 h; Tocris, Ellisville, MO), APV (20 μM, 72 h; Tocris), MPEP (10–50 μM, 16 or 72 h; Tocris), or LY367385 (100 μM, 16 h; Tocris) was added to the culture media of live neurons where indicated, and the conditioned media-containing drugs were replaced every 24 h. For staining, live neurons were incubated with rabbit antibody against the N-terminal of rat GluR1 (5 μg/ml; Calbiochem, San Diego, CA) or mouse anti N-terminal of GluR2 (2 μg/ml; Chemicon, Temecula, CA) for 10 min at 37°C (5% CO2), followed by DHPG (50 μM; Tocris) treatment for 15 min at 37°C (5% CO2) where indicated. Anisomycin (20 μM, 30 min; Sigma–Aldrich, St. Louis, MO), puromycin (100 μM, 30 min; Sigma–Aldrich), actinomycin D (25 μM, 30 min; Sigma–Aldrich), or cycloheximide (60 μM, 60 min; Sigma–Aldrich/Fluka) was added to the media before and during DHPG treatment where indicated. Neurons were fixed under nonpermeabilizing condition by incubating in 4% formaldehyde/4% sucrose/1× PBS (pH 7.2) for 30 min, and the surface GluR1 or GluR2 were saturated with Alexa 555-conjugated secondary antibody (Invitrogen/Molecular Probes, San Diego, CA) in ADB (0.1% BSA/4% normal goat serum/1× PBS) for 1 h. Neurons were then permeabilized for 1.5 min in methanol (−20°C) and incubated with mouse anti-FMRP antibody (1:100; gift of J. L. Mandel, Hopitaux Universitaires de Strasbourg, Strasbourg, France) or rabbit anti-Synapsin I (1:500; Chemicon International, Temecula, CA) in ADB for 1 h. FMRP and internalized GluR1 were visualized by incubation with Alexa 647-conjugated anti-mouse and Alexa 488-conjugated anti-rabbit secondary antibodies (Invitrogen/Molecular Probes) for 1 h. Last, neurons were incubated with Hoechst 33342 for nuclear staining and rinsed in 1× PBS. For fixed-cell staining, using the same reagent and condition described earlier, neurons were fixed for 30 min, labeled with rabbit anti-GluR1 for 1 h, rinsed, and saturated with secondary antibody.
Imaging, Quantification, and Data Analysis.
IF images were obtained by using a ×63 objective on an LSM 510 META confocal microscopy (Carl Zeiss, Thornwood, NY) at a constant setting through all of the experiments by using Zeiss software (Carl Zeiss). Obtained .lsm files were subjected to quantification with ImageJ software (37). The three thickest dendrites per neuron and 10 neurons per sample were chosen for quantification of IF signals. Total IF intensities for FMRP, surface, and internalized GluR1 were analyzed within a defined region of interest (ROI) traced along a dendrite and selected 10 μm from the cell body for proximal or secondary dendrites. Mean IF intensity was calculated by dividing the total IF intensity by area of the ROI. After subtraction of a background signal adjacent to each ROI, ratio of i/t GluR1 was calculated as follows: i/t GluR1 = mean intensity of internalized GluR1/(mean intensity of surface GluR1 + mean intensity of internalized GluR1). First, to normalize FMRP signals, mean IF signal intensities of individual dendrites in control and si-fmr1-transfected neurons were determined as the raw values. Second, the highest signal intensity in all those dendrites was set as the 100% value. Third, all of the raw values were corrected as the relative ratio to the 100% value. Calculated ratios and percentages of IF signals were graphed and statistically analyzed by using two-tailed ANOVA with α values of 0.05 by using Microsoft Excel software (Microsoft, Redmond, WA).
Piecewise Linear Regression Analyses.
To examine the effects of FMRP on GluR1, we used piecewise regression models (38) assuming one knot, which was selected based on minimization of the Akaike Information Criterion (39). We implemented these models by using SAS software (SAS Institute, Cary, NC).
Supplementary Material
Acknowledgments
We thank the S.T.W. Laboratory and Dr. Thomas Glover for helpful discussion, Tom Warren and Julie Mowrey for assistance, and Dr. Kate Garber and Morna Ikeda for proofreading of the manuscript. Confocal microscopy and image analysis was done in the Neuronal Imaging Core Facility. This work was supported by National Institutes of Health Grants NS051127 (to G.J.B.), HD20521 (to S.T.W.), and HD24064 (to S.T.W.) and a Fragile X Research Foundation grant.
Abbreviations
- FXS
fragile X syndrome
- FMRP
fragile X mental retardation protein
- KO
knockout
- LTD
long-term depression
- IF
immunofluorescence
- DHPG
(RS)-3,5-dihydroxyphenylglycine
- i/t GluR1
ratio of internalized GluR1 in total GluR1
- MPEP
2-methyl-6-phenylethynyl-pyridine
- ROI
region of interest.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0707484104/DC1.
References
- 1.Malenka RC, Bear MF. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Bredt DS, Nicoll RA. Neuron. 2003;40:361–379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- 3.Malenka RC. Ann NY Acad Sci. 2003;1003:1–11. doi: 10.1196/annals.1300.001. [DOI] [PubMed] [Google Scholar]
- 4.Carroll RC, Beattie EC, von Zastrow M, Malenka RC. Nat Rev Neurosci. 2001;2:315–324. doi: 10.1038/35072500. [DOI] [PubMed] [Google Scholar]
- 5.Chang EH, Savage MJ, Flood DG, Thomas JM, Levy RB, Mahadomrongkul V, Shirao T, Aoki C, Huerta PT. Proc Natl Acad Sci USA. 2006;103:3410–3415. doi: 10.1073/pnas.0507313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrison PJ, McLaughlin D, Kerwin RW. Lancet. 1991;337:450–452. doi: 10.1016/0140-6736(91)93392-m. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Pelletier MR, Perez Velazquez JL, Carlen PL. Mol Cell Neurosci. 2002;19:138–151. doi: 10.1006/mcne.2001.1085. [DOI] [PubMed] [Google Scholar]
- 8.O'Donnell WT, Warren ST. Annu Rev Neurosci. 2002;25:315–338. doi: 10.1146/annurev.neuro.25.112701.142909. [DOI] [PubMed] [Google Scholar]
- 9.Antar LN, Bassell GJ. Neuron. 2003;37:555–558. doi: 10.1016/s0896-6273(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 10.Bear MF, Huber KM, Warren ST. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Garber K, Smith KT, Reines D, Warren ST. Curr Opin Genet Dev. 2006;16:270–275. doi: 10.1016/j.gde.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Antar LN, Dictenberg JB, Plociniak M, Afroz R, Bassell GJ. Genes Brain Behav. 2005;4:350–359. doi: 10.1111/j.1601-183X.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- 13.Huber KM, Kayser MS, Bear MF. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- 14.Huber KM, Gallagher SM, Warren ST, Bear MF. Proc Natl Acad Sci USA. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou L, Antion MD, Hu D, Spencer CM, Paylor R, Klann E. Neuron. 2006;17:441–454. doi: 10.1016/j.neuron.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Koekkoek SK, Yamaguchi K, Milojkovic BA, Dortland BR, Ruigrok TJ, Maex R, De Graaf W, Smit AE, VanderWerf F, Bakker CE, et al. Neuron. 2005;47:339–352. doi: 10.1016/j.neuron.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Xiao MY, Zhou Q, Nicoll RA. Neuropharmacology. 2001;41:664–671. doi: 10.1016/s0028-3908(01)00134-4. [DOI] [PubMed] [Google Scholar]
- 18.Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF. Nat Neurosci. 2001;4:1079–1085. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- 19.Carroll RC, Beattie EC, Xia H, Luscher C, Altschuler Y, Nicoll RA, Malenka RC, von Zastrow M. Proc Natl Acad Sci USA. 1999;96:14112–14117. doi: 10.1073/pnas.96.24.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin JW, Ju W, Foster K, Lee SH, Ahmadian G, Wyszynski M, Wang YT, Sheng M. Nat Neurosci. 2000;3:1282–1290. doi: 10.1038/81814. [DOI] [PubMed] [Google Scholar]
- 21.Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, Malenka RC. Nat Neurosci. 2000;3:1291–1300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- 22.Ehlers MD. Neuron. 2000;28:511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- 23.Schwarz DS, Hutvagner G, Du T, Xu Z, Aronin N, Zamore PD. Cell. 2003;115:199–208. doi: 10.1016/s0092-8674(03)00759-1. [DOI] [PubMed] [Google Scholar]
- 24.Nosyreva ED, Huber KM. J Neurophysiol. 2006;95:3291–3295. doi: 10.1152/jn.01316.2005. [DOI] [PubMed] [Google Scholar]
- 25.Carroll FY, Stolle A, Beart PM, Voerste A, Brabet I, Mauler F, Joly C, Antonicek H, Bockaert J, Muller T, et al. Mol Pharmacol. 2001;59:965–973. [PMC free article] [PubMed] [Google Scholar]
- 26.Chuang SC, Zhao W, Bauchwitz R, Yan Q, Bianchi R, Wong RK. J Neurosci. 2005;25:8048–8055. doi: 10.1523/JNEUROSCI.1777-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfeiffer BE, Huber KM. J Neurosci. 2007;27:3120–3130. doi: 10.1523/JNEUROSCI.0054-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giuffrida R, Musumeci S, D'Antoni S, Bonaccorso CM, Giuffrida-Stella AM, Oostra BA, Catania MV. J Neurosci. 2005;25:8908–8916. doi: 10.1523/JNEUROSCI.0932-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenough WT, Klintsova AY, Irwin SA, Galvez R, Bates KE, Weiler IJ. Proc Natl Acad Sci USA. 2001;98:7101–7106. doi: 10.1073/pnas.141145998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antar LN, Afroz R, Dictenberg JB, Carroll RC, Bassell GJ. J Neurosci. 2004;24:2648–2655. doi: 10.1523/JNEUROSCI.0099-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Neuropharmacology. 2005;49:1053–1066. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 32.McBride SM, Choi CH, Wang Y, Liebelt D, Braunstein E, Ferreiro D, Sehgal A, Siwicki KK, Dockendorff TC, Nguyen HT, et al. Neuron. 2005;45:753–764. doi: 10.1016/j.neuron.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 33.Tucker B, Richards RI, Lardelli M. Hum Mol Genet. 2006;15:3446–3458. doi: 10.1093/hmg/ddl422. [DOI] [PubMed] [Google Scholar]
- 34.Volk LJ, Daly CA, Huber KM. J Neurophysiol. 2006;95:2427–2438. doi: 10.1152/jn.00383.2005. [DOI] [PubMed] [Google Scholar]
- 35.Goslin K, Asmussen H, Banker G. In: Culturing Nerve Cells. Banker G, Goslin K, editors. Cambridge, MA: MIT Press; 1998. pp. 339–370. [Google Scholar]
- 36.Craig AM. In: Culturing Nerve Cells. Banker G, Goslin K, editors. Cambridge, MA: MIT Press; 1998. pp. 79–111. [Google Scholar]
- 37.Abramoff MD, Magelhaes PJ, Ram SJ. Biophot Internat. 2004;11:36–42. [Google Scholar]
- 38.Ruppert D, Wand MP, Carroll RJ. Semiparametric Regression. Cambridge, UK: Cambridge Univ Press; 2003. [Google Scholar]
- 39.Akaike H. In: A Celebration of Statistics. Atkinson AC, Fienberg SE, editors. New York: Springer; 1985. pp. 1–24. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.