Abstract
The Saccharomyces cerevisiae phosphatidylcholine/phosphatidylinositol transfer protein Sec14p is required for Golgi apparatus-derived vesicular transport through coordinate regulation of phospholipid metabolism. Sec14p is normally essential. The essential requirement for SEC14 can be bypassed by inactivation of (i) the CDP–choline pathway for phosphatidylcholine synthesis or (ii) KES1, which encodes an oxysterol binding protein. A unique screen was used to determine genome-wide genetic interactions for the essential gene SEC14 and to assess whether the two modes of “sec14 bypass” were similar or distinct. The results indicate that inactivation of the CDP–choline pathway allows cells with inactivated SEC14 to live through a mechanism distinct from that of inactivation of KES1. We go on to demonstrate an important biological function of Kes1p. Kes1p regulates Golgi apparatus-derived vesicular transport by inhibiting the function of Pik1p-generated Golgi apparatus phosphatidylinositol-4-phosphate (PI-4P). Kes1p affects both the availability and level of Golgi apparatus PI-4P. A set of potential PI-4P-responsive proteins that include the Rab GTPase Ypt31p and its GTP exchange factor are described.
Keywords: KES1, Saccharomyces cerevisiae, SEC14, vesicular transport
The Golgi apparatus plays an essential role in the intracellular trafficking of proteins and membranes. Roles for specific lipids in Golgi apparatus-derived secretion were demonstrated in Saccharomyces cerevisiae, where it was determined that Sec14p, the major phosphatidylcholine (PC)/phosphatidylinositol (PI) transfer protein in these cells, was essential for vesicular transport from the Golgi apparatus (1, 2). Sec14p is thought to act as a biological sensor of lipid composition that regulates PC and PI metabolism in the Golgi apparatus. Although PC and PI metabolism are clearly linked to Golgi apparatus function through Sec14p, our knowledge of the role of its lipid effectors in the regulation of Golgi apparatus function is sparse.
Alleviation of the cell growth and secretion defects due to the loss of function of Sec14p can be bypassed by inactivation of five different genes, all of which participate in the regulation of lipid metabolism. The essential requirement for Sec14p can be bypassed by mutations in the following: CKI1, PCT1, or CPT1, the genes encoding the enzymes of CDP–choline pathway for PC synthesis (1); KES1, coding for an oxysterol binding protein family member that binds both sterols and PI 4-phosphate (PI-4P) (3–6); and SAC1, which codes for a PI-4P phosphatase (7). The function of phospholipids in Golgi apparatus secretion and its regulation by phospholipid transfer proteins is highly conserved, because knockdown of the mammalian Golgi apparatus-localized PI transfer protein Nir2 severely reduced Golgi apparatus-derived secretion and pharmacological inhibition of the CDP–choline pathway in cells with reduced Nir2 function restored Golgi apparatus vesicular transport (8).
In this study, we initially sought to determine whether the modes of regulation of (i) inhibition of PC synthesis versus (ii) Kes1p function on Sec14p-mediated Golgi apparatus vesicular transport were similar or distinct. To accomplish this, four separate genome-wide synthetic genetic array (SGA) screens were performed (9, 10). The SGA analyses determined that the mode of action of inactivation of the CDP–choline pathway for PC synthesis bypasses SEC14 function in a manner distinct from that of KES1. This screen also revealed that the Rab GTPase Ypt31p and nonessential components of its GTP exchange factor, the transport protein particle II (TRAPPII) complex (11, 12), are required for viability and Golgi apparatus vesicular transport in cells lacking Sec14p function but only when KES1 is functional. We go on to find that Kes1p is a key regulator of Golgi apparatus PI-4P level and availability.
Results
SGA Analysis of “sec14 Bypass” Reveals Distinct Networks.
SGA analysis is a genetic screening method that takes advantage of the existence of a set of yeast strains in which each of ≈5,000 nonessential yeast genes has been individually inactivated. SGA analysis allows for a systematic analysis of a phenotype, usually growth, when a query gene of interest is inactivated simultaneously in the same strain as every nonessential yeast gene (10). In addition, titratable promoters, mRNA destabilization, and hypomorphic alleles have recently been used to determine genetic interaction spectrums for essential genes (13, 14).
SGA analysis using genetic suppressors that allow for growth in the absence of a normally essential gene is a potential complementary approach for determining the genetic spectrum of an essential gene and is the only one that would use a completely inactivated allele. In addition, screening two different genes whose inactivation allows for life in the absence of a normally essential gene, termed “bypass” mutations, has the added utility of determining whether genetic bypasses work through similar or distinct modes of action. If the modes of action are the same, then each screen will identify the same set of genetic interactors. If each mode of bypass is distinct, then many of the genes required for life of each bypass strain will be different. This study assesses the function of an essential gene by using this approach.
Sec14p is thought to regulate PC and PI metabolism such that an appropriate Golgi apparatus phospholipid composition is maintained to facilitate vesicular transport. Knowledge of the precise lipid mediators regulated by Sec14p to sustain vesicular transport, and their protein effectors, is scant. In this study, the SEC14 gene was deleted in yeast strains containing an inactivated gene for the following: (i) CKI1, which encodes the choline kinase that catalyzes the first step in the CDP–choline pathway for PC synthesis (15); or (ii) KES1, a member of the oxysterol binding protein family that also binds phosphoinositides (3, 4). The sec14 cki1 and sec14 kes1 bypass strains were subjected to SGA analysis, as were cki1 and kes1 single mutant strains. The resulting ≈19,000 yeast strains were analyzed for growth defects. No genes were found to confer lethality to cki1 or kes1 cells; thus, every gene whose inactivation aggravated growth of the sec14 bypass strains was specific to the combined loss of function of SEC14 with CKI1 or KES1.
The SGA screens identified 21 genes specifically required for sec14 cki1 cell viability, 4 for sec14 kes1 cells, and 12 genes required for life of both strains (Fig. 1). According to the Saccharomyces Genome Database (www.yeastgenome.org), of the 21 genes specifically required for sec14 cki1 bypass, most have described roles in vesicular trafficking at the Golgi apparatus. This is consistent with a major function of Sec14p being the regulation of Golgi apparatus vesicular transport. Of the four genes whose inactivation specifically aggravated growth of sec14 kes1 cells, none has a known role in the regulation of Golgi apparatus vesicle transport nor do they appear to possess a common link. The genes required for growth of both the sec14 cki1 and sec14 kes1 strains were enriched in coding for proteins required for cell wall maintenance. Because Golgi apparatus to plasma membrane transport is required for effective delivery of cell wall synthesizing proteins to the plasma membrane and periplasm, the data imply that the genes common to both screens constitute a downstream point of convergence of Kes1p and the CDP–choline pathway at the plasma membrane. Because the majority of the genes isolated from the sec14 cki1 and sec14 kes1 genetic screens were different, PC synthesis and Kes1p regulate Golgi apparatus vesicular transport via different modes of action.
Fig. 1.
Interaction diagram for the genetic interactions discovered for the query strains sec14 cki1 and sec14 kes1. Genes are represented as nodes and interactions are represented as edges with genes colored based on Gene Ontology (www.geneontology.org) function: blue, metabolism; light purple, cell organization and biogenesis; dark purple, transport; pink, protein transport; yellow; budding; green, cell cycle; orange, protein biosynthesis; light blue, sporulation; cyan, transcription; gold, protein amino acid phosphorylation; gray, unknown. See SI Tables 1 and 2 for the strains used and function of each gene.
A Requirement for Kes1p for Function of the Rab GTPase Ypt31p and the TRAPPII Complex.
A subset of the genes isolated from the SGA screen were analyzed by construction of isogenic strains. We analyzed the specificity of the CDP–choline pathway for PC synthesis, versus KES1, for the requirement for the Rab GTPase Ypt31p and the TRAPPII complex. The TRAPPII complex possesses GTP exchange factor activity for Ypt31p and the highly similar Ypt32p (12, 16). Trs33p, Trs65p, and Trs85p comprise the entire set of nonessential components of the 10-subunit Golgi apparatus-associated TRAPPII complex and all three were isolated from the SGA screen as required for life of sec14 cki1, but not sec14 kes1, cells. Ypt31p and Ypt32p comprise an essential gene pair that regulates vesicle export and import at the Golgi apparatus (11). Ypt31p, but not Ypt32p, was isolated as from the SGA screen as required for life of sec14 cki1 cells.
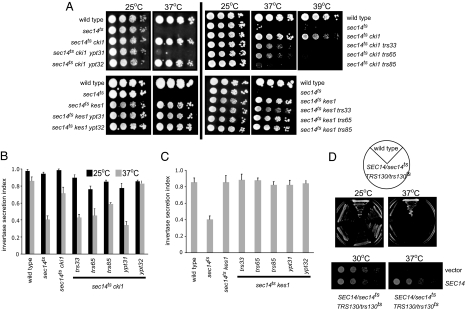
The YPT31, YPT32, TRS33, TRS65, or TRS85 gene was inactivated by one-step gene replacement in isogenic sec14ts cki1 and sec14ts kes1 strains. Inactivation of YPT31 in sec14ts cki1 cells resulted in severe growth and secretion defects at 37°C, whereas inactivation of YPT32 in sec14ts cki1 or sec14ts kes1 strains displayed no growth or secretion defects (Fig. 2A–C). Inactivation of the nonessential TRAPPII components TRS33, TRS65, or TRS85 reduced growth and secretion of sec14ts cki1 cells, but not sec14ts kes1 cells, when grown at the nonpermissive temperature for the sec14ts allele (Fig. 2 A–C). Importantly, inactivation of YPT31, TRS33, TRS65, or TRS85 reduced growth or secretion only when Sec14p was function was reduced.
Fig. 2.
Requirement of the Rab GTPase Ypt31p and TRAPPII components in Sec14p function. (A) The isogenic sec14ts yeast strains indicated were grown to logarithmic phase, 10-fold serial dilutions were spotted on YEPD agar plates, and cells were incubated for 48–72 h at 25, 37, or 39°C. (B and C) The secretion index was determined by comparing the activity of extracellular invertase to total invertase. Data are the mean of at least three independent experiments ± SE. (D) Diploid SEC14/sec14ts TRS130/trs130ts cells were grown in culture at 25°C. Identical cell numbers were serial diluted, plated, and grown at 25 or 37°C. Growth of five different SEC14/sec14ts TRS130/trs130ts strains is shown. A SEC14/sec14ts TRS130/trs130ts diploid strain was transformed with a low-copy plasmid carrying the SEC14 gene, or empty vector, grown in culture to logarithmic phase at 25°C; identical cell numbers were serial diluted; and growth at 30 and 37°C was determined.
Cells lacking TRS33, TRS65, or TRS85 obtained by SGA analysis or tetrad dissection in combination with ablated SEC14 and CKI1 genes resulted in more dramatic defects in growth than in cells containing the sec14ts allele. This is likely due to residual Sec14p activity at 37°C for this allele (our unpublished results). To further assess the genetic interaction between Sec14p and the TRAPPII complex, a temperature-sensitive allele for an essential member of the TRAPPII complex, trs130ts, was used (17). Diploid cells were constructed in which there was one wild-type and one thermosensitive allele for both SEC14 and TRS130 (SEC14/sec14ts TRS130/trs130ts). All of the cells heterozygous for both SEC14 and TRS130 displayed growth defects at the nonpermissive temperature of 37°C (Fig. 2D). Diploid cells heterozygous for either SEC14 or TRS130 alone were not temperature sensitive (unpublished result), nor were SEC14/sec14ts TRS130/trs130ts cells transformed with low-copy SEC14 (Fig. 2D). This synthetic haploinsufficiency further indicates the combined functions of TRAPPII and Sec14p are important for secretion from the Golgi apparatus.
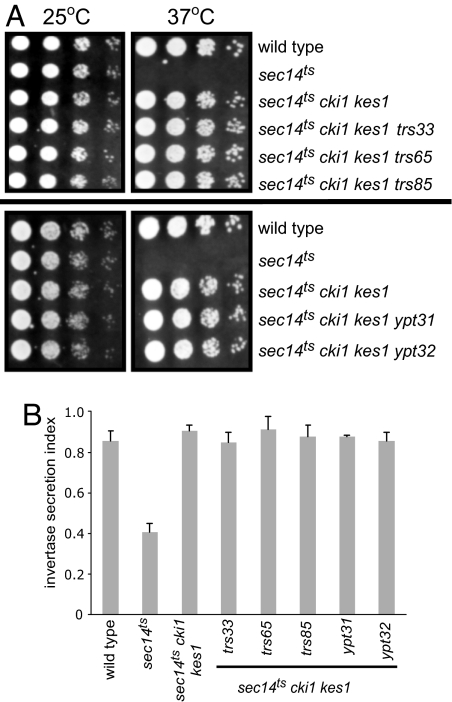
In sec14 cki1 cells, Kes1p is present, implying that Ypt31p/TRAPPII function is downstream of Kes1p and that Kes1p acts as an inhibitor of Ypt31p/TRAPPII function. If this is true, then inactivation of KES1 in sec14 cki1 ypt31 or sec14 cki1 trappII cells could restore growth. Inactivation of KES1 in sec14ts cki1 ypt31 and sec14ts cki1 trappII cells reestablished growth and vesicular transport from the Golgi apparatus when cells were grown at the nonpermissive temperature for the sec14ts allele (Fig. 3). This also demonstrates that the contributions of PC synthesis and Kes1p to inhibition of Sec14p-mediated vesicle transport are additive.
Fig. 3.
Requirement of KES1 for growth and secretion in sec14 cki1 cells. (A) The isogenic yeast strains indicated were grown to logarithmic phase, and 10-fold serial dilutions of identical cell numbers were spotted on YEPD agar plates and incubated for 48–72 h at 25°C or 37°C. (B) The secretion index was determined by measuring the activity of the extracellular invertase compared with the total invertase (extracellular plus internal) of cells grown at 25°C or shifted to 37°C for 2 h. Data are the mean of at least three independent experiments ± SE.
To order the events linking Sec14p function with activation of the Ypt31p/TRAPPII complex, we overexpressed Ypt31p, or an activated “GTP-locked” Ypt31p, in cells containing sec14ts or trs130ts alleles. Consistent with the TRAPPII complex being the GTP exchange factor for Ypt31p (12, 16), increased expression of Ypt31p or GTP-locked Ypt31p allowed for growth of trs130ts cells at nonpermissive temperatures (Fig. 4). However, overexpression of Ypt31p or GTP-locked Ypt31p did not support growth of sec14ts cells. This is consistent with Sec14p lying upstream of Ypt31p and regulating the production or action of a factor required for the function of the Ypt31/32p pair or the TRAPPII complex.
Fig. 4.
Activated Ypt31p does not rescue growth of Sec14p-deficient cells. The sec14ts and trs130ts strains were transformed with the indicated plasmids and grown to mid-log phase at 25°C in minimal medium. Equal numbers of cells were harvested by centrifugation, and serial dilutions were spotted on plates and incubated for 3 days at the indicated temperatures.
Kes1p Regulates Pik1p-Generated Golgi Apparatus PI-4P Function.
A subset of genes obtained from our SGA analysis that were found to be required for life of sec14 cki1 cells, YPT31 (Rab GTPase), TRS33 and TRS65 (TRAPP complex subunits), ARL1 (Arf-like GTPase), VPS1 (dynamin-like GTPase), VPS51 (Golgi apparatus-associated retrograde protein subunit), and VPS4 (AAA-type ATPase), comprised 8 of the 15 genes required for Golgi apparatus-derived membrane trafficking in cells containing a partially functional PI 4-kinase (PIK1) allele (17). Pik1p is a Golgi apparatus- and nuclear-resident PI 4-kinase whose inactivation results in trans-Golgi apparatus defects very similar to those observed on loss of function of Sec14p (18–20). Because KES1 is present in sec14 cki1 cells, and Kes1p has been demonstrated to bind PI-4P (3, 21), we reasoned that Kes1p and Pik1p may coordinately control vesicular transport through regulation of Golgi apparatus PI-4P function. We were unable to find any other genetic or protein interaction networks within the literature or available databases that significantly overlapped with our gene set.
If Kes1p works to oppose Pik1p function, then inactivation of KES1 should at least partially restore growth to cells with compromised Pik1p function. Inactivation of KES1 in pik1ts cells restored the growth and secretion defects associated with inactivation of Pik1p function (Fig. 5 A and B). Inactivation of KES1 in cells containing a temperature-sensitive allele of STT4, the PI 4-kinase responsible for the synthesis of PI-4P at the plasma membrane, did not restore growth to stt4ts cells grown at the nonpermissive temperature (Fig. 5A), indicating that Kes1p does not regulate the function of Stt4p-generated PI-4P. Recently, Pik1p has been determined to have essential functions in both the Golgi apparatus and the nucleus (19). The essential protein Frq1p aids in targeting Pik1p to the Golgi apparatus. We established that kes1 frq1 cells were viable, and expression of KES1 from a regulated promoter in kes1 frq1 cells resulted in cell death (Fig. 5C). A Kes1p-GFP chimera primarily localized with the Golgi apparatus marker Sec7p-DsRed (Fig. 5D). Thus, Kes1p acts directly at the Golgi apparatus as an inhibitor of Pik1p-derived PI-4P function.
Fig. 5.
Kes1p regulates the function of Pik1p-generated Golgi apparatus PI-4P. (A) Cells were grown to mid-log phase at 25°C in minimal medium, and equal numbers of cells were serial diluted, spotted onto plates, and incubated for 3 days at the indicated temperatures. (B) Wild-type, pik1ts, and pik1ts kes1 cells were grown to mid-log phase at 25°C, labeled with [35S]methionine/cysteine for 10 min at 25°C, and chased with 10 mM cysteine and 10 mM methionine for 30 min at 37°C. Proteins in the medium were precipitated, washed, and separated by SDS/PAGE. The gel was exposed to a phosphorimager screen. (C) A kes1 frq1 yeast strain transformed with a plasmid expressing KES1 under control of a galactose inducible promoter was grown at 30°C to mid-log phase and washed, and identical numbers of cells were serial diluted and spotted onto plates containing glucose (to repress expression of KES1) or galactose (to induce expression of KES1). Cells were incubated for 3 days at 30°C. (D) BY4741 cells with genomically tagged KES1-GFP were transformed with a plasmid expressing the Golgi apparatus marker Sec7-dsRed and grown to early-log phase at 25°C, and live cells were visualized through GFP, red fluorescent protein, or differential interference contrast filters. Images were captured by using a Zeiss AxioCam HR using Zeiss AxioVision 4.1 software.
Kes1p Regulates Pik1p-Produced PI-4P Level.
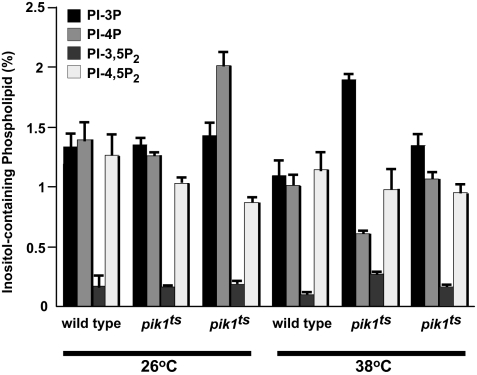
To further assess the role of Kes1p in the regulation of Golgi apparatus PI-4P function, we determined whether Kes1p affected the levels of Pik1p-generated PI-4P. The levels of phosphoinositides were determined in wild-type, pik1ts, and pik1ts kes1 cells grown at the permissive and nonpermissive temperatures for the pik1ts allele. Consistent with previous results (18), PI-4P levels decreased to ≈50% of wild type in pik1ts cells grown at the nonpermissive temperature. Inactivation of KES1 in pik1ts cells increased PI-4P levels slightly above wild type when cells were grown at the semipermissive temperature for the pik1ts allele. PI-4P levels were restored to wild type when pik1ts cells were grown at the nonpermissive temperature for pik1ts function (Fig. 6). The levels of the other phosphoinositides were not altered, indicating specificity of Kes1p for regulation of PI-4P mass.
Fig. 6.
The level of PI-4P is regulated by Kes1p. SEY6210 (wild type), AAY104 (pik1ts), and GFY201 (AAY104 kes1::KanMx) cells were grown to mid-log phase at 26°C in inositol-free medium and labeled with myo-[2-3H]inositol for 50 min at 26°C or 38°C. Phosphoinositides were extracted, deacylated, separated by high-performance liquid chromatography, and quantitated by using an on-line radiometric detector. Data are expressed as percentages of the total number of counts and are expressed as mean ± SE of three separate experiments. PI-3,5P2, phosphatidylinositol-3,5-bisphosphate; PI-4,5P2, phosphatidylinositol-4,5-bisphosphate.
Golgi Apparatus PI-4P Accessibility Is Determined by Kes1p.
We further postulated that Kes1p could also regulate Golgi apparatus PI-4P function through its PI-4P binding ability. In part, this was due to the observation that there are ≈32,000 molecules of Kes1p per cell (22) and that PI-4P accounts for 0.03% (≈80,000 of the ≈300 million) phospholipid molecules per yeast cell. Because Pik1p generates almost one-half of cellular PI-4P (18), or ≈40,000 PI-4P molecules per cell, there is enough Kes1p to sequester a large portion of Golgi apparatus PI-4P. The PI-4P binding PH (pleckstrin homology) domain of Osh2p in tandem with GFP is a specific marker of PI-4P pools (23), and we used this reporter to determine the role of Kes1p in regulating Golgi apparatus PI-4P availability.
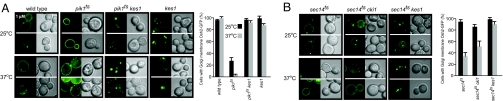
The low-level expression of Osh2p-GFP used for PI-4P reporting did not affect growth of any of the yeast strains tested. In wild-type cells, PI-4P was present at the plasma membrane with some punctate staining previously determined to be Golgi apparatus, whereas in pik1ts cells there was very little PI-4P reporter at the Golgi apparatus even when these cells were grown at a semipermissive temperature for the pik1ts allele, and when shifted to a nonpermissive temperature Golgi apparatus staining decreased to negligible levels (Fig. 7A). Inactivation of KES1 in wild-type cells resulted in an increase in PI-4P signal at the Golgi apparatus and also resulted in reestablishment of the PI-4P probe to the Golgi apparatus in pik1ts cells (Fig. 7A). The data demonstrate that Kes1p regulates Golgi apparatus PI-4P accessibility.
Fig. 7.
Availability of Golgi apparatus PI-4P is a requirement for Pik1p- and Sec14p-dependent Golgi apparatus vesicular transport. (A and B) Cells were transformed with a PI-4P reporter that contains the PH domain of Osh2p in tandem with GFP expressed from a low-copy plasmid. Cells were grown to early-log phase at 25°C. A subset of cells was shifted to 37°C for 15 min before visualization. Cells were visualized by using GFP or differential interference contrast filters using a plan-neofluor ×100 oil immersion objective lens. Quantitation of Golgi apparatus staining from three independent experiments with at least 100 cells documented for each strain per experiment is expressed as mean ± SE.
Kes1p and Sec14p Coordinately Regulate Golgi Apparatus PI-4P Availability.
It is known that inactivation of KES1 alleviates growth and secretion defects associated with loss of function of Sec14p. In addition, the level of cellular PI-4P is reduced by ≈50% in cells with reduced Sec14p function (18, 24). If Sec14p regulates Golgi apparatus PI-4P function, then Golgi apparatus PI-4P availability should be decreased in sec14ts and sec14ts cki1 cells (because Kes1p is present), but the same as wild type in sec14ts kes1 cells. Golgi apparatus and plasma membrane localization of the PI-4P reporter was similar to wild-type cells for sec14ts, sec14ts cki1, and sec14ts kes1 cells grown at the permissive temperature of 25°C. After shift to the nonpermissive temperature for the sec14ts allele, the PI-4P reporter was no longer apparent at the Golgi apparatus of sec14ts and sec14ts cki1 cells, whereas its appearance was similar to wild type in sec14ts kes1 cells (Fig. 7B). These data are also consistent with the SGA analysis of the sec14 cki1 and sec14 kes1 cells that predicted different modes of action for regulation of Sec14p-mediated vesicle transport by the CDP–choline pathway for PC synthesis and Kes1p. Kes1p regulates Golgi apparatus-derived vesicular transport through inhibition of Golgi apparatus PI-4P function, whereas the CDP–choline pathway regulates Golgi apparatus secretion independent of Golgi apparatus PI-4P function.
Discussion
Here, we show that a major function of Kes1p is to regulate vesicular transport from the Golgi apparatus through modulation of Golgi apparatus PI-4P availability and level (Fig. 8). The genome-wide SGA screen described uses a completely inactivated allele of an essential gene. This was facilitated by the knowledge that inactivation of either KES1, or any of the genes that comprise the CDP–choline pathway for PC synthesis, allows yeast cells to grow in the absence of the normally essential SEC14 gene. This approach allowed for the determination of important new functions for genes/proteins that regulate lipid metabolism and identified 36 new genes whose inactivation restore cell growth to cells where SEC14 is inactivated.
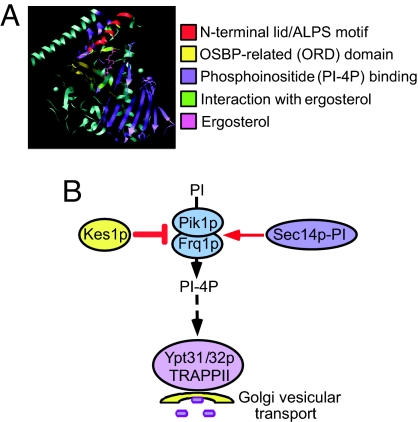
Fig. 8.
Model of Kes1p regulation of Golgi apparatus PI-4P. (A) Structure of Kes1p with ergosterol (4) with known domains colored as indicated. (B) Pik1p synthesizes PI-4P at the Golgi apparatus. Golgi apparatus PI-4P levels are regulated by Kes1p and Sec14p, although whether either protein directly affects Pik1p function has yet to be determined. Kes1p also affects Golgi apparatus PI-4P availability by limiting the ability of proteins to assemble/deassemble by competing for PI-4P binding. Genetic evidence from this study places Ypt31/32p and its GTP exchange factor, the TRAPPII complex, downstream of Sec14p, implying that their function is regulated by Golgi apparatus lipid composition. ALPS, ArfGAP1 lipid packing sensor; OSBP, oxysterol binding protein.
PI-4P is enriched in Golgi apparatus and plasma membranes. Golgi apparatus PI-4P is synthesized by Pik1p, whereas plasma membrane PI-4P is synthesized by Stt4p (18). PI-4P levels are also regulated by the PI/PC transfer protein Sec14p through an unknown mechanism. Cells containing pik1ts, sec14ts, or stt4ts alleles possess PI-4P levels that are ≈50% wild type when grown at the restrictive temperature (18, 24). Kes1p is a member of the oxysterol binding protein family that has been demonstrated to directly bind PI-4P under several different in vitro experimental conditions, and in cells the association of Kes1p with the Golgi apparatus is decreased when Pik1p function is reduced (3). Inactivation of KES1 restored growth to cells with reduced Pik1p or Sec14p function, but not Stt4p function. Inactivation of KES1 also restored PI-4P levels to wild type in pik1ts and sec14ts cells. A fluorescent PI-4P reporter determined that Pik1p and Sec14p both contribute to the synthesis of Golgi apparatus PI-4P because Golgi apparatus PI-4P staining was significantly decreased in cells with reduced Pik1p or Sec14p function. Inactivation of KES1 in these cells reestablished the accessibility of the PI-4P reporter for the Golgi apparatus. As Golgi apparatus PI-4P availability becomes critical (as is found in cells where Sec14p or Pik1p function is compromised), the ability of Kes1p to inhibit Golgi apparatus PI-4P synthesis, as well as directly bind PI-4P, results in an inhibition of vesicular transport.
The proposed role for Kes1p acting specifically on Golgi apparatus PI-4P function is consistent with the observation that overexpression of a Golgi apparatus-anchored derivative of Pik1p was toxic when expressed in cells where KES1 was inactivated, whereas overexpression of a nuclear localized version of Pik1p was not. In a separate study, overexpression of nuclear-localized Pik1p restored viability to pik1ts kes1 cells grown at the nonpermissive temperature for the pik1ts allele, whereas pik1ts kes1 cells were inviable when either wild-type Pik1p or Golgi apparatus-anchored Pik1p was overexpressed (T. Strahl and J. Thorner, personal communication). The combined results clearly indicate that Kes1p counteracts the function of Pik1p- and Frq1p-generated PI-4P at the Golgi apparatus, but does not affect the function of Pik1p in the nucleus.
Yeast contain six other members of the oxysterol binding protein family besides Kes1p (Osh1p to -3p and Osh5p to -7p; Kes1p is also referred to as Osh4p). None of the Osh family members is essential when inactivated alone. Simultaneous ablation of all seven family members is lethal with described defects including reduced endocytosis and alterations in sterol distribution (25). Kes1p is unique within the yeast oxysterol binding protein family in that only inactivation of Kes1p is capable of bypassing the requirement for Sec14p function. Kes1p is also the only member of the oxysterol binding protein family whose structure has been determined. The crystal structure revealed binding of a single sterol molecule within the core of Kes1p (4). Sterol binding by Kes1p was promiscuous because several sterols, including ergosterol, cholesterol, and several oxysterols, were capable of binding. Pure Kes1p was demonstrated to extract sterols from model membranes in minutes, whereas sterol transfer to acceptor membranes required time frames in the order of hours, implying that extraction is much more efficient than transfer (26). Whether Kes1p transfers sterols in vivo is still unclear. Indeed, there is no correlation between mutations in Kes1p that reduced sterol binding with the ability to effect growth of (i) a yeast strain in which all members of the oxysterol binding protein family in yeast had been genetically inactivated except for KES1 or (ii) a sec14ts kes1 strain (4).
A further level of regulation with respect to Kes1p and lipid association is the finding of an ArfGAP1 lipid packing sensor motif present in the N-terminal 29 aa of Kes1p (27). This motif allows for association of proteins with highly curved, versus flat, membranes. This is consistent with a role for Kes1p in the regulation of vesicle transport. Because this motif was unresolved in the crystal structure of unliganded Kes1p, but formed the predicted helix motif in liganded Kes1p, sterol binding may serve as a switch to allow for Kes1p to associate with vesicles. This remains to be tested experimentally. Ergosterol binding has been demonstrated to influence the function of two other yeast oxysterol binding protein family members, Osh6p and Osh7p, by regulating their association with the AAA ATPase Vps4p (28). Proteins that directly interact with Kes1p await identification.
The functions of the oxysterol-binding protein family members are not clear. Proposed roles for Kes1p include regulation of nonvesicular sterol transfer between membranes (26) and vesicle transport at the trans-Golgi apparatus. The genome-wide screen performed clearly identified a role for Kes1p in the regulation of vesicular transport at the Golgi apparatus. Evidence for Kes1p in nonvesicular sterol transfer between membranes was not apparent. We propose a model whereby Kes1p binds to Golgi apparatus membranes through its affinity for PI-4P. Kes1p binding to Golgi apparatus PI-4P inhibits the synthesis of PI-4P by Pik1p, as well as the availability of Golgi apparatus PI-4P for binding by other proteins. The identities of in vivo Golgi apparatus PI-4P binding proteins that are affected by Kes1p, as well as protein binding partners for Kes1p, now need to be determined.
Materials and Methods
SGA Analysis.
Genome-wide synthetic lethal screens were conducted for the following query strains, cki1 (CMY209), kes1 (CMY210), sec14 cki1 (CMY212), and sec14 kes1 (CMY213). The nourseothricin resistant (natR) marked query strains (both single and double deletion mutants) were constructed and analyzed by using SGA analysis (10), modified as described in supporting information (SI) Materials and Methods.
Cell Growth Determinations.
Cells were grown in yeast extract/peptone/dextrose (YEPD) medium to logarithmic phase, and cell concentration was estimated by measuring absorbance at 600 nm. Cells were washed and resuspended to a concentration of 0.4 absorbance units at 600 nm, and a series of serial dilutions were plated on YEPD agar plates and allowed to grow for 2–3 days at the indicated temperatures. Plates were imaged by using a Versa Doc Imaging System (Bio-Rad, Hercules, CA) apparatus.
Invertase Assays.
Cells were grown to logarithmic phase in YEPD at 25°C, washed with YEPD containing 0.1% glucose, and cultured in YEPD containing 0.1% glucose at either 25° or 37°C for 2 h. Secretion index was defined as the ratio of external (secreted) invertase activity compared with the total (secreted plus intracellular) activity (1).
Hsp150 Secretion.
Wild-type, pik1ts, and pik1ts kes1 cells were grown to mid-logarithmic phase in minimal medium lacking cysteine and methionine, and labeled with Expre35S35S Mix for 10 min at 25°C. Cells were chased by incubation in medium containing 10 mM cysteine and 10 mM methionine for 30 min at 37°C. Precipitated proteins in the medium were washed twice with acetone and separated by SDS/PAGE. The gel was exposed to a phosphorimager screen.
Microscopy.
The PI-4P reporter contains the PH domain of Osh2p in tandem with GFP (23). In experiments using temperature-sensitive alleles, cells containing the PI-4P reporter were shifted to 37°C for 15 min before visualization. Cells were grown to early-log phase at 25°C, and live cells were visualized through GFP, red fluorescent protein, or differential interference contrast filters using a Zeiss (Oberkochen, Germany) Axiovert 200 M microscope fitted with a plan-neofluor ×100 oil immersion objective lens. Images were captured by using a Zeiss AxioCam HR and by using Zeiss AxioVision 4.1 software.
Analysis of Phosphoinositides.
Phosphoinositide levels were determined as described in ref. 29.
Supplementary Material
Acknowledgments
We thank Nava Segev (University of Illinois, Chicago, IL), Scott Emr (Cornell University), and Tim Levine (University College London, London, U.K.) for yeast strains and plasmids and Scott Emr, Vytas Bankaitis, Christopher Beh, and J. Pedro Fernández-Murray for helpful suggestions. This work was supported by a Canada Research Chair award and a National Sciences Engineering Research Council operating grant (to C.R.M.).
Abbreviations
- PC
phosphatidylcholine
- PI
phosphatidylinositol
- PI-4P
phosphatidylinositol-4-phosphate
- SGA
synthetic genetic array
- TRAPPII
transport protein particle II
- YEPD
yeast extract/peptone/dextrose.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.D.Y. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0705571104/DC1.
References
- 1.Cleves AE, McGee TP, Whitters EA, Champion KM, Aitken JR, Dowhan W, Goebl M, Bankaitis VA. Cell. 1991;64:789–800. doi: 10.1016/0092-8674(91)90508-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bankaitis VA, Malehorn DE, Emr SD, Greene R. J Cell Biol. 1989;108:1271–1281. doi: 10.1083/jcb.108.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li X, Rivas MP, Fang M, Marchena J, Mehrotra B, Chaudhary A, Feng L, Prestwich GD, Bankaitis VA. J Cell Biol. 2002;157:63–77. doi: 10.1083/jcb.200201037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Im YJ, Raychaudhuri S, Prinz WA, Hurley JH. Nature. 2005;437:154–158. doi: 10.1038/nature03923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang M, Kearns BG, Gedvilaite A, Kagiwada S, Kearns M, Fung MK, Bankaitis VA. EMBO J. 1996;15:6447–6459. [PMC free article] [PubMed] [Google Scholar]
- 6.Fairn GD, McMaster CR. Biochem J. 2005;387:889–896. doi: 10.1042/BJ20041915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rivas MP, Kearns BG, Xie Z, Guo S, Sekar MC, Hosaka K, Kagiwada S, York JD, Bankaitis VA. Mol Biol Cell. 1999;10:2235–2250. doi: 10.1091/mbc.10.7.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Litvak V, Dahan N, Ramachandran S, Sabanay H, Lev S. Nat Cell Biol. 2005;7:225–234. doi: 10.1038/ncb1221. [DOI] [PubMed] [Google Scholar]
- 9.Fairn GD, McMaster CR. Methods. 2005;36:102–108. doi: 10.1016/j.ymeth.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Tong AH, Evangelista M, Parsons AB, Xu H, Bader GD, Page N, Robinson M, Raghibizadeh S, Hogue CW, Bussey H, et al. Science. 2001;294:2364–2368. doi: 10.1126/science.1065810. [DOI] [PubMed] [Google Scholar]
- 11.Benli M, Doring F, Robinson DG, Yang X, Gallwitz D. EMBO J. 1996;15:6460–6475. [PMC free article] [PubMed] [Google Scholar]
- 12.Morozova N, Liang Y, Tokarev AA, Chen SH, Cox R, Andrejic J, Lipatova Z, Sciorra VA, Emr SD, Segev N. Nat Cell Biol. 2006;8:1263–1269. doi: 10.1038/ncb1489. [DOI] [PubMed] [Google Scholar]
- 13.Schuldiner M, Collins SR, Thompson NJ, Denic V, Bhamidipati A, Punna T, Ihmels J, Andrews B, Boone C, Greenblatt JF, et al. Cell. 2005;123:507–519. doi: 10.1016/j.cell.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 14.Davierwala AP, Haynes J, Li Z, Brost RL, Robinson MD, Yu L, Mnaimneh S, Ding H, Zhu H, Chen Y, et al. Nat Genet. 2005;37:1147–1152. doi: 10.1038/ng1640. [DOI] [PubMed] [Google Scholar]
- 15.McMaster CR, Bell RM. J Biol Chem. 1994;269:14776–14783. [PubMed] [Google Scholar]
- 16.Jones S, Newman C, Liu F, Segev N. Mol Biol Cell. 2000;11:4403–4411. doi: 10.1091/mbc.11.12.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sciorra VA, Audhya A, Parsons AB, Segev N, Boone C, Emr SD. Mol Biol Cell. 2005;16:776–793. doi: 10.1091/mbc.E04-08-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Audhya A, Foti M, Emr SD. Mol Biol Cell. 2000;11:2673–2689. doi: 10.1091/mbc.11.8.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strahl T, Hama H, DeWald DB, Thorner J. J Cell Biol. 2005;171:967–979. doi: 10.1083/jcb.200504104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walch-Solimena C, Novick P. Nat Cell Biol. 1999;1:523–525. doi: 10.1038/70319. [DOI] [PubMed] [Google Scholar]
- 21.Knodler A, Mayinger P. BioTechniques. 2005;38:858, 860, 862. doi: 10.2144/05386BM02. [DOI] [PubMed] [Google Scholar]
- 22.Ghaemmaghami S, Huh WK, Bower K, Howson RW, Belle A, Dephoure N, O'Shea EK, Weissman JS. Nature. 2003;425:737–741. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- 23.Roy A, Levine TP. J Biol Chem. 2004;279:44683–44689. doi: 10.1074/jbc.M401583200. [DOI] [PubMed] [Google Scholar]
- 24.Hama H, Schnieders EA, Thorner J, Takemoto JY, DeWald DB. J Biol Chem. 1999;274:34294–34300. doi: 10.1074/jbc.274.48.34294. [DOI] [PubMed] [Google Scholar]
- 25.Beh CT, Rine J. J Cell Sci. 2004;117:2983–2996. doi: 10.1242/jcs.01157. [DOI] [PubMed] [Google Scholar]
- 26.Raychaudhuri S, Im YJ, Hurley JH, Prinz WA. J Cell Biol. 2006;173:107–119. doi: 10.1083/jcb.200510084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drin G, Casella JF, Gautier R, Boehmer T, Schwartz TU, Antonny B. Nat Struct Mol Biol. 2007;14:138–146. doi: 10.1038/nsmb1194. [DOI] [PubMed] [Google Scholar]
- 28.Wang P, Zhang Y, Li H, Chieu HK, Munn AL, Yang H. EMBO J. 2005;24:2989–2999. doi: 10.1038/sj.emboj.7600764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stefan CJ, Audhya A, Emr SD. Mol Biol Cell. 2002;13:542–557. doi: 10.1091/mbc.01-10-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.