Abstract
Endocannabinoids acting on CB1 cannabinoid receptors are involved in short- and long-term depression of synaptic transmission. The aim of the present study was to determine which endocannabinoid, anandamide or 2-arachidonoylglycerol (2-AG), is involved in depolarization-induced suppression of inhibition (DSI) in the cerebellar cortex, which is the most widely studied form of short-term depression. Depolarization of Purkinje cells in the mouse cerebellum led to an increase in intracellular calcium concentration and to suppression of the inhibitory input to these neurons (i.e. DSI occurred). Orlistat and RHC80267, two blockers of sn-1-diacylglycerol lipase, the enzyme catalysing 2-AG formation, abolished DSI by acting downstream of calcium influx. In contrast, DSI occurred also in the presence of a phospholipase C inhibitor. Intact operation of the calcium-dependent messengers calmodulin and Ca2+–calmodulin-dependent protein kinase II were necessary for DSI. DSI was potentiated by an inhibitor of the main 2-AG-degrading enzyme, monoacylglycerol lipase. Interference with the anandamide metabolizing enzyme, fatty acid amide hydrolase, did not modify DSI. Thus, three kinds of observations identified 2-AG as the endocannabinoid involved in DSI in the mouse cerebellum: DSI was abolished by diacylglycerol lipase inhibitors; DSI was potentiated by a monoglyceride lipase inhibitor; and DSI was not changed by an inhibitor of fatty acid amide hydrolase. Further experiments indicated that 2-AG is the endocannabinoid mediating short-term retrograde signalling also at other synapses: orlistat abolished DSI in the rat cerebellum, DSI in the mouse substantia nigra pars reticulata and depolarization-induced suppression of excitation in the mouse cerebellum.
The Gαi/o protein-coupled cannabinoid CB1 receptor is probably the most abundant G protein-coupled receptor in the central nervous system. It is the primary neuronal target of the phytocannabinoid Δ9-tetrahydrocannabinol and the endogenous cannabinoids (endocannabinoids) (Howlett et al. 2002; Pertwee, 2005). Activation of CB1 receptors leads to presynaptic inhibition of synaptic transmission in many regions of the central and peripheral nervous system (Freund et al. 2003; Szabo & Schlicker, 2005).
It has recently been discovered that endocannabinoids and CB1 receptors play a physiological role in both short- and long-term regulation of the strength of synaptic transmission (for review see Alger, 2002; Wilson & Nicoll, 2002; Freund et al. 2003; Gerdeman & Lovinger, 2003; Diana & Marty, 2004; Chevaleyre et al. 2006).
One form of short-term synaptic depression is triggered by depolarization of postsynaptic neurons. Endocannabinoids mediate depolarization-induced suppression of inhibitory synapses (depolarization-induced suppression of inhibition, DSI) (Llano et al. 1991; Ohno-Shosaku et al. 2001; Varma et al. 2001; Wilson & Nicoll, 2001; Diana et al. 2002) and depolarization-induced suppression of excitatory synapses (depolarization-induced suppression of excitation, DSE) (Kreitzer & Regehr, 2001; Ohno-Shosaku et al. 2002). DSI and DSE are thought to be due to retrograde synaptic signalling involving the following steps: depolarization of postsynaptic neurons elicits an increase in intracellular calcium concentration; the elevated calcium levels trigger endocannabinoid synthesis; the released endocannabinoids diffuse to presynaptic axon terminals where they inhibit GABA (DSI) or glutamate (DSE) release by acting on presynaptic CB1 receptors. Another form of endocannabinoid-mediated short-term retrograde synaptic signalling is triggered by activation of certain Gαq/11 protein-coupled receptors on postsynaptic neurons (Maejima et al. 2001, 2005; Kim et al. 2002; Brown et al. 2003; Galante & Diana, 2004; Marcaggi & Attwell, 2005). Retrogradely diffusing endocannabinoids are also involved in long-term synaptic depression evoked by moderate- to high-frequency stimulation of presynaptic axons (for example, Gerdeman et al. 2002; Robbe et al. 2002; Chevaleyre & Castillo, 2003).
The two best-characterized endocannabinoids are anandamide (Devane et al. 1992; Di Marzo et al. 1994) and 2-arachidonoylglycerol (2-AG) (Mechoulam et al. 1995; Stella et al. 1997). The significance of the more recently discovered endocannabinoids noladin ether, virodhamine and N-arachidonoyl-dopamine is not yet clear (for review on endocannabinoids see Mechoulam et al. 1998; Piomelli, 2003; Di Marzo, 2005).
Although the role of endocannabinoids in retrograde synaptic signalling is well established, the knowledge on the chemical identity of the endocannabinoid involved and the chain of events leading to enhanced endocannabinoid release is limited. Thus, the endocannabinoid mediating DSI and DSE has been determined only in the hippocampus (Kim & Alger, 2004; Makara et al. 2005; Straiker & Mackie, 2005). The aim of the present study was to determine which of the two major endocannabinoids, anandamide or 2-AG, is involved in DSI at interneuron–Purkinje cell synapses in the cerebellar cortex. To this end, we studied the effects of inhibitors of endocannabinoid formation and degradation on DSI. In addition, involvement of intracellular messengers in the stimulation of endocannabinoid synthesis was also studied. Some of the findings have been published in abstract form (Urbanski et al. 2005; Szabo et al. 2005).
Methods
The experiments conformed to the European Community law regulating the use of animals in biomedical research. The methods were similar to those previously described (Bisogno et al. 2003; Szabo et al. 2004; Freiman et al. 2006).
Endocannabinoid production in N18TG2 neuroblastoma cells
Confluent N18TG2 cells (DSMG, Braunschweig, Germany) were incubated for 20 min at 37°C in Dulbecco's modified Eagle's medium supplemented with fetal bovine serum (10%) and 6-thioguanine (10−4m), according to the manufacturer's instructions. Endocannabinoid production was stimulated by addition of the calcium ionophore ionomycin (3 × 10−6m) to the incubation medium. After stimulation, cells plus media were extracted with chloroform/methanol (2/1; v/v). Extracts were purified by open bed chromatography and 2-AG and anandamide were quantified by isotope dilution liquid chromatography – atmospheric pressure chemical ionization – mass spectrometry (Bisogno et al. 2003).
Brain slices
Ten- to 18-day-old NMRI mice were anaesthetized with isoflurane (> 3%) and decapitated. The brains were rapidly removed and placed in ice-cold artificial cerebrospinal fluid (ACSF) of the following composition (mm): NaCl 126, NaH2PO4 1.2, KCl 3, MgCl2 5, CaCl2 1, NaHCO3 26, glucose 20 and sodium lactate 4, pH 7.3–7.4 (after the solution was gassed with 95% O2–5% CO2). In most experiments, 250 μm thick sagittal slices of the cerebellar vermis were cut. In a few experiments, 300 μm thick oblique sagittal slices containing the caudate-putamen and the substantia nigra pars reticulata (SNR) or 300 μm thick coronal slices containing the hippocampus were prepared. Some experiments were carried out on 250 μm thick sagittal cerebellar slices prepared from 10- to 18-day-old Wistar rats. After cutting, the slices were stored in a Gibb chamber containing ACSF of the following composition (mm): NaCl 126, NaH2PO4 1.2, KCl 3, MgCl2 1, CaCl2 2.5, NaHCO3 26, glucose 10 and sodium lactate 4, pH 7.3–7.4. For patch clamping, brain slices were superfused at 20–24°C at a flow rate of 1.5 ml min−1 with ACSF of the following composition (mm): NaCl 126, NaH2PO4 1.2, KCl 3, MgCl2 1, CaCl2 2.5, NaHCO3 26 and glucose 10, pH 7.3–7.4.
Patch clamping
Neurons in slices were visualized with infrared video microscopy. Patch-clamp recordings were obtained with an EPC-9 amplifier under the control of TIDA software (HEKA Elektronik, Lambrecht, Germany). Series resistance compensation of 50% was usually applied. Series resistance was measured before and after recordings and experiments with major changes in series resistance (> 20%) were discarded. Inhibitory postsynaptic currents (IPSCs), spontaneous IPSCs (sIPSCs), miniature IPSCs (mIPSCs) and excitatory postsynaptic currents (EPSCs) were recorded at a holding potential of −70 mV with pipettes containing (mm): CsCl 147, MgCl2 1, Hepes 10, EGTA 1, ATP-Na2 4, GTP-Na 0.4 and N-ethyl-lidocaine chloride 2, pH 7.4. For IPSC and sIPSC recording, the superfusion ACSF contained the glutamate receptor antagonists 6,7-dinitroquinoxaline-2,3-dione (DNQX) (10−5m) and DL-2-amino-5-phosphonopentanoic acid (AP5) (2.5 × 10−5m). EPSCs were recorded in the presence of the GABAA receptor antagonist bicuculline (2 × 10−5m) and the GABAB receptor antagonist CGP55845 (10−6m). sIPSCs and mIPSCs were detected with the MiniAnalysis software (version 6.0.1; Synaptosoft, Decatur, GA, USA); the program allowed analysis of complex peaks consisting of several inhibitory currents. Amplitudes and recording times of sIPSCs and mIPSCs were transferred from MiniAnalysis to SigmaPlot (SPSS, Chicago, IL, USA), and a program written by us in Sigmaplot calculated cumulative amplitudes by summating amplitudes of all events within 10 s periods.
Fluorescence measurement of calcium concentrations in Purkinje cells
In addition to the intracellular solution used for recording postsynaptic currents, the patch pipette contained the low affinity calcium indicator Oregon green 488 BAPTA-5 N (Kd for calcium, 2 × 10−5m; final concentration, 2 × 10−4m). Fluorescence intensity in Purkinje cells was determined with an imaging system consisting of: Polychrome IV monochromatic light source, a cooled IMAGO VGA CCD camera and TILLvision imaging software (TILL Photonics, Gräfelfing, Germany). For measuring Oregon green fluorescence, the excitation wave length of the monochromatic light source was adjusted to 495 nm, and a 505DRLP dichroic filter and a 535AF45 bandpass emission filter were used (Omega Optical, Brattleboro, VT, USA). Fluorescence changes were evaluated in regions of interest (ROIs). Fluorescence values were corrected for background fluorescence. For further evaluation, ratios between stimulation-evoked fluorescence changes (ΔF) and baseline fluorescence measured immediately before stimulation (F0) were calculated (ΔF/F0 ratios).
Protocols and statistics
Recordings started 15 (electrophysiological recordings) or 40 min (calcium imaging) after establishment of the whole-cell configuration. DSI or DSE were usually elicited twice in a neuron, and the results of both trials were included in the evaluation. DSI and DSE were quantified by expressing cumulative sIPSC amplitudes as percentages of the initial reference value (PRE) (see Fig. 1). Amplitudes of electrical stimulation-evoked eIPSCs and eEPSCs were also expressed as percentages of the PRE value. Effects of drugs on PRE were systematically evaluated but reported only if they were significant. Solvent and drug superfusion is indicated in the figures; drug superfusion started at least 20 min before the respective DSI recordings. Drug-treated groups were compared with control groups generated during the same time period and receiving the appropriate solvent. Means ± s.e.m. are given throughout. Non-parametric statistical tests were used to identify significant differences. The two-tailed Mann–Whitney U test was used for comparisons between groups; significant differences are indicated by asterisks. The two-tailed Wilcoxon signed rank test was used for comparisons within groups (versus PRE); significant differences are indicated by filled symbols. P < 0.05 was taken as the limit of statistical significance, and only this level is indicated, even if P was < 0.01 or < 0.001. For multiple comparisons, the P levels were corrected according to Bonferroni.
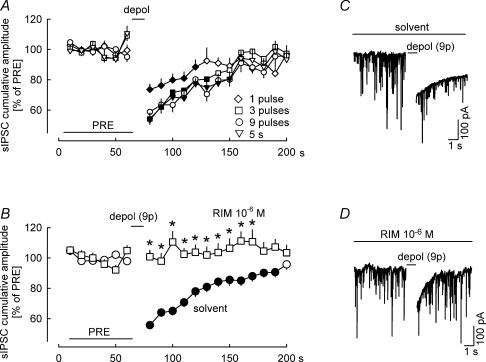
Figure 1. Characterization of depolarization-induced suppression of inhibition (DSI) in the cerebellar cortex.
Spontaneous IPSCs (sIPSCs) were recorded in Purkinje cells. Cumulative amplitudes of sIPSCs were calculated for 10 s periods and expressed as percentages of the initial reference value PRE. A, after PRE, one, three or nine depolarizing pulses (from −70 to 0 mV for 100 ms) were applied at 1 Hz; one group was depolarized once (from −70 to 0 mV for 5 s). All four depolarization protocols led to suppression of the cumulative amplitude of sIPSCs (i.e. DSI occurred). Means ± s.e.m. of 23 (one pulse), 25 (three pulses), 24 (nine pulses) and 25 (5 s) experiments. B, effects of nine depolarizing pulses (from −70 to 0 mV for 100 ms) at 1 Hz in the presence of solvent and the CB1 receptor antagonist rimonabant (RIM). Prevention of DSI by RIM points to involvement of endocannabinoids and CB1 receptors. Means ± s.e.m. of 59 (solvent) and 23 (RIM) experiments. C and D, 20 s tracings showing the effect of depolarization on sIPSCs in the presence of solvent and RIM. Note that the depolarization induces not only a suppression of sIPSCs, but also a slow inward current. This latter is thought to be due to a calcium-induced chloride current. A filled symbol indicates significant difference versus PRE and asterisk indicates significant difference versus solvent (P < 0.05).
Drugs
Drugs were obtained from the following sources. Calbiochem (Darmstadt, Germany): 1,6-bis(cyclohexy-loximinocarbonylamino) hexane (RHC80267), autocamtide-2 related inhibitory peptide myristoylated, fluphenazine-mustard dihydrochloride and 2-[N-(2-hyd-roxyethyl)]-N-(4-methoxybenzenesulphonyl)]-amino-N-(4-chlorocinnamyl)-N-methylbenzylamine (KN-93); Cayman Chemicals (Ann Arbor, MI, USA): anandamide, 2-arachidonoyl glycerol, 3′carbamoyl-biphenyl-3-ylcyclohexylcarbamate (URB597) and arachidonoyl trifluoromethylketone (ATFMK); Endocannabinoid Research Group, Institute of Biomolecular Chemistry (Pozzuoli, Italy): arachidonoyl 5-hydroxytryptamine (AA-5-HT); F. Hoffman-La Roche (Basel, Switzerland): orlistat (also called tetrahydrolipstatin); Pharmacia & Upjohn (Kalamazoo, MI, USA): 4-[5-(4-chlorophenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benzenesulphonamide (SC-58236); Sanofi-Aventis (Chilly Mazarin, France): rimonabant (previously called SR141716A); Sigma (Milan, Italy): ionomycin.
Anandamide, 2-AG, ATFMK, fluphenazine-mustard, orlistat, RHC80267, rimonabant, SC-58236 and URB597 were dissolved in dimethylsulphoxide (DMSO), and stock solutions were stored at −32°C. Further dilutions were made with superfusion buffer; the final concentration of DMSO in the superfusion fluid was ≤ 1 ml l−1. Control solutions (‘solvent’ in the figures) always contained the appropriate concentrations of DMSO. AA-5-HT was dissolved in ethanol; the final concentration of ethanol in the superfusion buffer was 1 ml l−1 (this was also the concentration of ethanol in the appropriate ‘solvent’ control group).
Results
Characterization of the effects of orlistat on diacylglycerol lipase
2-AG is produced by cleavage of diacylglycerol by the recently cloned sn-1-diacylglycerol lipase that exists in two isoforms, α and β (Bisogno et al. 2003). This enzyme, as well as other diacylglycerol lipases, is inhibited by RHC80267 (Stella et al. 1997; Moriyama et al. 1999). A more potent and selective inhibitor of diacylglycerol lipase, orlistat, was identified by Bisogno et al. (2003); see also Bisogno et al. 2006. In the first series of experiments, we characterized the effects of orlistat on enzymes involved in endocannabinoid formation and degradation and on CB1 and CB2 cannabinoid receptors. Orlistat inhibited diacylglycerol lipase α with an IC50 value of 6 × 10−8m (Table 1). Monoglyceride lipase (the enzyme hydrolysing 2-AG), N-acylphosphatidylethanolamine-selective phospholipase D (the enzyme producing anandamide), fatty acid amide hydrolase (the enzyme hydrolysing anandamide) and triglyceride lipase were inhibited only at much higher concentrations (Table 1). Moreover, orlistat possessed only low affinity for CB1 and CB2 cannabinoid receptors (Table 1). Thus, orlistat selectively inhibits diacylglycerol lipase, and not other components of the endocannabinoid system. RHC80267, the compound which is traditionally used to inhibit diacylglycerol lipase, inhibited diacylglycerol lipase with an IC50 value of 6.5 ± 0.12 × 10−5m (n = 3). Thus, it is clearly less potent than orlistat.
Table 1.
Effects of orlistat on proteins of the endocannabinoid system and triglyceride lipase
| Source of protein | IC50 or Ki* value (10−6m) | |
|---|---|---|
| aDiacylglycerol lipase α | human enzyme expressed in COS cells | 0.06 ± 0.02 |
| bMonoglyceride lipase | COS cells | > 25 |
| cN-acylphosphatidylethanolamine-selective phospholipase D | rat enzyme expressed in HEK-293 cells | 10 ± 1 |
| dFatty acid amide hydrolase | rat brain | > 25 |
| eTriglyceride lipase | rat liver | 1.0 ± 0.2 |
| fCB1 cannabinoid receptor | human receptor expressed in COS cells | 4.0 ± 0.8* |
| gCB2 cannabinoid receptor | human receptor expressed in COS cells | > 25* |
Means ± s.e.m. of n = 4 experiments.
Diacylglycerol lipase α activity was studied in membranes (100 μg protein) using 1-[14C]oleoyl-2-arachidonoyl-glycerol (1 mCi mmol−1, 2.5 × 10−5m) as substrate (see Bisogno et al. 2003).
Monoglyceride lipase activity was studied in the cytosol derived from the 10 000 g fraction of homogenates (100 μg protein) using 2-[3H]arachidonoyl-glycerol (1.0 mCi mmol−1, 2.5 × 10−5m) as substrate (see Bisogno et al. 2003). Wild-type COS cells express high amounts of monoglyceride lipase mRNA and the indicated fraction exhibits the highest monoglyceride lipase activity (T. Bisogno & V. Di Marzo, unpublished observations).
N-Acylphosphatidylethanolamine-selective phospholipase D activity was studied in membrane fractions from confluent HEK-293 cells transiently transfected with this enzyme using phosphatidylethanolamine-N-[3H]-arachidonoyl (200 Ci mmol−1, 10−4m) as substrate (see Okamoto et al. 2004). Lipids were extracted with two volumes of chloroform/methanol (2/1; v/v). The extracts were purified by thin-layer chromatography (TLC) on silica plates eluted with the organic phase from a mixture of isooctane/ethylacetate/water/acetic acid (50/110/100/20; v/v/v/v). Radioactivity in TLC bands corresponding to anandamide or arachidonic acid was determined.
Fatty acid amide hydrolase activity was studied in membranes prepared from rat brain using [14C]anandamide (5 mCi mmol−1, 5 × 10−6m) as substrate (see Cascio et al. 2004).
Triglyceride lipase was studied in debris-free homogenates of rat liver (100 μg protein) using 1,2,3-[14C]oleoyl-glycerol as substrate (100 mCi mmol−1, 2.5 × 10−5m). After extraction and TLC, radioactivity in TLC bands corresponding to [14C]oleic acid was determined.
Binding assays on membranes from COS cells transfected with either recombinant human CB1 or CB2 receptors were performed as previously described (see Appendino et al. 2005).
We also studied the effects of orlistat on endogenous 2-AG and anandamide production in N18TG2 neuroblastoma cells incubated for 20 min at 37°C. Orlistat did not affect the calcium-independent basal 2-AG and anandamide production (not shown). 2-AG production in the presence of the calcium ionophore ionomycin (3 × 10−6m) was 51 ± 3 pmol mg−1 lipid extract (n = 3). Orlistat (10−6m) strongly suppressed the ionomycin-induced 2-AG production (suppression by 84 ± 2%; n = 3; P < 0.05). Anandamide production in the presence of ionomycin (3 × 10−6m) was 1.7 ± 0.5 pmol mg−1 lipid extract (n = 3). Orlistat (10−6m) did not affect the ionomycin-induced anandamide production (change by 0 ± 11%; n = 3; P > 0.05). Thus, orlistat selectively inhibits 2-AG production also in intact neuronal cells.
Characterization of DSI
Cerebellar cortical Purkinje cells were patch clamped, and spontaneous inhibitory postsynaptic currents (sIPSCs) were recorded at a membrane potential of −70 mV. Cumulative amplitudes of sIPSCs were calculated for 10 s periods and expressed as percentages of the initial reference value PRE (see Fig. 1A). Four kinds of depolarization protocols were applied after PRE (Fig. 1A). Either a series of one, three or nine depolarizing pulses (from −70 to 0 mV for 100 ms) were applied at 1 Hz, or the neuron was depolarized once (from −70 to 0 mV for 5 s). Already a single 100 ms depolarizing step led to significant suppression of sIPSCs (i.e. DSI occurred). The suppressions following three and nine 100 ms depolarizing pulses and the 5 s depolarization were greater and lasted longer. We decided to carry out all following experiments in Purkinje cells using nine depolarizing pulses – a stimulus causing maximal DSI.
Depolarization by nine pulses in the presence of solvent suppressed sIPSCs to 56% of PRE and the suppression lasted about 120 s (Fig. 1B and C). No DSI was observed in the presence of the CB1 cannabinoid receptor antagonist rimonabant (10−6m; Fig. 1B and D). This observation points to involvement of endocannabinoids and CB1 receptors in DSI. Altogether, the properties of DSI in our preparation were similar to those observed previously in Purkinje cells (e.g. Diana et al. 2002; Brenowitz & Regehr, 2003; Szabo et al. 2004).
Interference with 2-AG production
Effects of diacylglycerol lipase inhibitors on DSI
In order to identify the endocannabinoid involved in DSI, at first we employed inhibitors of 2-AG production. Our hypothesis was that if DSI is mediated by 2-AG, then diacylglycerol lipase inhibitors should attenuate DSI. When orlistat (10−7–10−5m) was superfused, it blocked DSI in a concentration-dependent manner (Fig. 2A and B), with full blockade occurring at the concentration of 10−5m (Fig. 2B). Superfusion of another inhibitor of diacylglycerol lipase, RHC80267 (10−4m), also significantly attenuated DSI (Fig. 2C). The less than full blockade by RHC80267 is probably due to the low potency of this inhibitor, as described above.
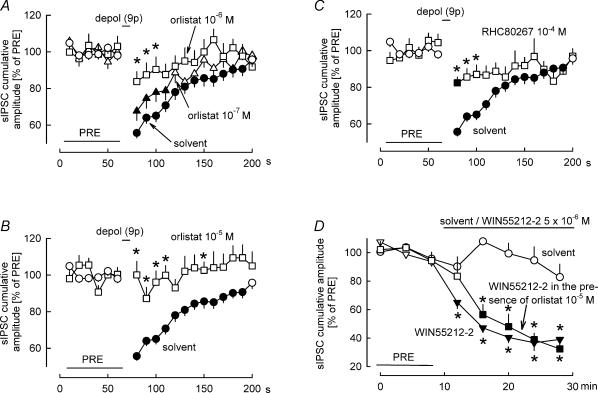
Figure 2. Inhibition of diacylglycerol lipase attenuates DSI.
A–C, in the presence of solvent, nine depolarizing pulses (from −70 to 0 mV for 100 ms) at 1 Hz elicited DSI. The diacylglycerol lipase inhibitor orlistat blocked DSI in a concentration-dependent manner, with full blockade occurring at the highest concentration. RHC80267, another diacylglycerol lipase inhibitor, also attenuated DSI. Means ± s.e.m. of 59 (solvent), 22 (orlistat 10−7m), 21 (orlistat 10−6m), 25 (orlistat 10−5m) and 27 (RHC80267 10−4m) experiments. D, solvent and the CB1/CB2 cannabinoid receptor agonist WIN55212-2 were superfused as indicated. In one of the WIN55212-2 groups, orlistat (10−5m) was present in the superfusion buffer during the entire experiment. WIN55212-2 inhibited sIPSCs, and this inhibition was not affected by orlistat. Means ± s.e.m. of 10 (solvent), 10 (WIN55212-2) and eight (WIN55212-2 in the presence of orlistat) experiments. A–D, filled symbol indicates significant difference versus PRE and asterisk indicates significant difference versus solvent (P < 0.05).
Orlistat possesses some affinity for CB1 receptors (but this affinity is much lower than the affinity of the compound for diacylglycerol lipase; see Table 1). Theoretically, both agonist as well as antagonist activity at CB1 receptors would attenuate DSI. Orlistat caused a minor increase in sIPSCs before DSI induction (during the PRE period indicated in Fig. 2): the cumulative amplitude of sIPSCs was 12123 ± 716 pA (10 s)−1 in the solvent-treated group and 13705 ± 951 pA (10 s)−1 in the group receiving orlistat (10−5m) (P = 0.04). This observation excludes the possibility that orlistat acted as a CB1 receptor agonist (CB1 agonists inhibit sIPSCs recorded in Purkinje cells; Takahashi & Linden, 2000). We also carried out experiments to find out whether orlistat is able to block CB1 receptors. The mixed CB1/CB2 receptor agonist WIN55212-2 (5 × 10−6m) lowered the cumulative amplitude of sIPSCs recorded in Purkinje cells (Fig. 2D). The inhibitory effect of WIN55212-2 was not changed in slices that were superfused in addition with orlistat (10−5m; Fig. 2D), excluding the possibility that orlistat behaved as a CB1 receptor antagonist in our preparation. Thus, it is very unlikely that orlistat prevented DSI by acting on or inhibiting CB1 receptors.
As described above, orlistat (10−5m) slightly enhanced the cumulative amplitude of sIPSCs before DSI (from 12123 ± 716 to 13705 ± 951 pA (10 s)−1; an increase of 13%). It is theoretically possible – although not likely – that these orlistat-evoked sIPSCs cannot be suppressed by depolarization. In such a case, orlistat would apparently decrease the calculated DSI even without true interference with diacylglycerol lipase. According to the data shown in Fig. 2B, DSI was 44% in the solvent-treated group. Assuming the above mechanism, orlistat (10−5m) could decrease DSI to 39%, even without true interference with diacylglycerol lipase. However, the complete abolishment of DSI by orlistat (10−5m; Fig. 2B) obviously cannot be explained by this mechanism.
It must be noted that although relatively high concentrations of orlistat and RHC80267 were necessary to inhibit diacylglycerol lipase in brain slices, this does not mean that these drugs lost their selectivity for this enzyme. In the case of the highly lipophilic cannabinoids (e.g. WIN55212-2, and Δ9-tetrahydrocannabinol), the concentrations eliciting effects in brain slices are always several orders of magnitude higher than the affinities determined on cell monolayers or membrane preparations. This is probably due to poor diffusion into the brain slice (see Brown et al. 2004). Very probably, the same holds true for orlistat and RHC80267 (octanol/buffer partition coefficients, > 1000 and ∼25 000, respectively).
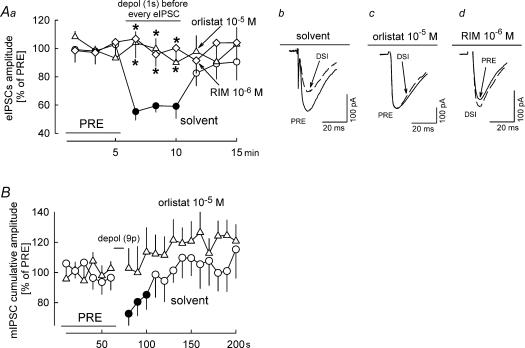
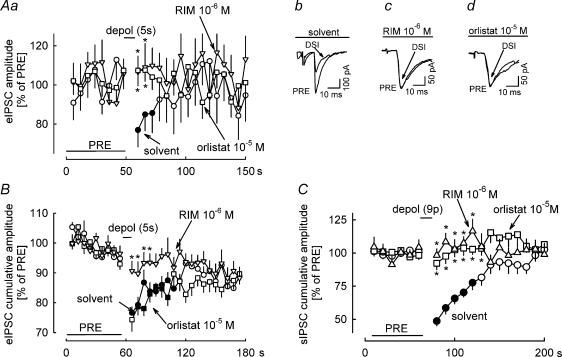
Depolarization-induced suppression of sIPSCs in the cerebellar cortex is due to endocannabinoid-mediated inhibition of the firing of interneurons, inhibition of voltage-dependent calcium channels in the GABAergic axon terminals and inhibition of the vesicle release machinery in the axon terminals (Kreitzer et al. 2002; Diana & Marty, 2003). For obtaining more information on the involvement of diacylglycerol lipase in these processes, we tested the effect of orlistat on depolarization-induced suppression of electrically evoked IPSCs (eIPSCs) and miniature IPSCs (mIPSCs) (Fig. 3). eIPSCs in Purkinje cells were evoked by electrical stimulation in the outer molecular layer. Three seconds before each eIPSC, Purkinje cells were depolarized from −70 to 0 mV for 1 s. This depolarization led to suppression of eIPSCs (eIPSC-DSI) in the presence of solvent (Fig. 3Aa and Ab). Rimonabant (10−6m) and orlistat prevented eIPSC-DSI (Fig. 3Aa, Ac and Ad). mIPSCs were isolated in the presence of tetrodotoxin (3 × 10−7m). Nine depolarizing pulses (from −70 to 0 mV for 100 ms) at 1 Hz suppressed the following mIPSCs (i.e. mIPSC-DSI occurred) (Fig. 3B). No mIPSC-DSI occurred in the presence of orlistat (10−5m) (Fig. 3B). Blockade of eIPSC-DSI and mIPSC-DSI by orlistat indicates that the endocannabinoid inhibiting GABA release from the axon terminals was produced by diacylglycerol lipase. Blockade of eIPSC-DSI and mIPSC-DSI by orlistat also excludes the possibility that orlistat attenuated sIPSC-DSI (Fig. 2A and B) by enhancing the number of sIPSCs which cannot be suppressed by depolarization.
Figure 3. Effects of orlistat on depolarization-induced suppression of electrical stimulation-evoked IPSCs (eIPSC-DSI) and on depolarization-induced suppression of miniature IPSCs (mIPSC-DSI).
Aa, eIPSCs in Purkinje cells were elicited by electrical stimulation in the outer molecular layer of the cerebellar cortex at 0.05 Hz and five consecutive eIPSCs were averaged. The experiments had three 5 min phases: an initial reference period (PRE); a DSI period, during which every eIPSCs was preceded by depolarization of the postsynaptic Purkinje cell from −70 to 0 mV for 1 s (the depolarization started 3 s before the eIPSC); and the final period without postsynaptic depolarizations. In the presence of solvent, DSI occurred. DSI was abolished in the presence of rimonabant (RIM) and orlistat. Means ± s.e.m. of seven (solvent), 10 (RIM) and 15 (orlistat) experiments. Ab–Ad, Original tracings showing DSI in the presence of solvent and abolishment of DSI by RIM and orlistat. B, mIPSC-DSI was studied essentially as sIPSC-DSI (see Fig. 2), except that tetrodotoxin (3 × 10−7m) was present during the entire experiment for isolation of mIPSCs. In the presence of solvent, nine depolarizing pulses (from −70 to 0 mV for 100 ms) at 1 Hz elicited DSI. DSI was abolished in the presence of orlistat. Means ± s.e.m. of 15 (solvent) and 16 (orlistat) experiments. A and B, filled symbol indicates significant difference versus PRE and asterisk indicates significant difference versus solvent (P < 0.05).
Involvement of phospholipase C in DSI
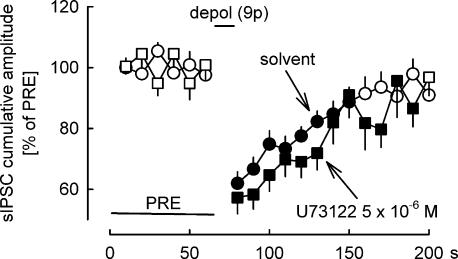
The results shown above suggest that production of 2-AG from diacylglycerol by diacylglycerol lipase is essential for DSI. Diacylglycerols which serve as a biosynthetic precursors for 2-AG can be produced via phospholipase C-dependent or -independent pathways (Sugiura et al. 2002). The next question was whether the operation of phospholipase C is necessary for DSI. We studied, how DSI is influenced by the phospholipase C inhibitor U73122 (Fig. 4). U73122 was superfused at a concentration of 5 × 10−6m; at this concentration it elicits effects in brain slices (Chevaleyre & Castillo, 2003; Galante & Diana, 2004; Edwards et al. 2006). However, U73122 (5 × 10−6m) did not inhibit DSI (Fig. 4).
Figure 4. Effect of the inhibition of phospholipase C on DSI.
DSI was elicited by nine depolarizing pulses (from −70 to 0 mV for 100 ms) at 1 Hz. DSI was not influenced by the phospholipase C inhibitor U73122. Means ± s.e.m. of 20 (solvent) and 16 (U73122) experiments. A filled symbol indicates significant difference versus PRE (P < 0.05).
Effects of orlistat on depolarization-induced intracellular calcium concentration increases
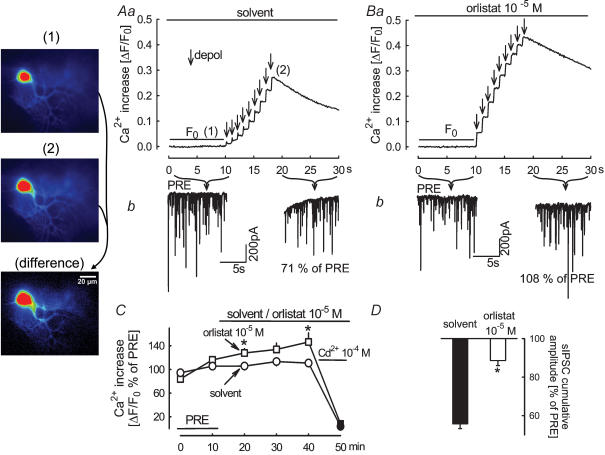
A necessary event involved in DSI is calcium influx into the postsynaptic neuron, triggered by the depolarization (Ohno-Shosaku et al. 2001; Wilson & Nicoll, 2001; Brenowitz & Regehr, 2003; Engler et al. 2006). We tested whether orlistat interferes with calcium influx into the Purkinje cells. The patch pipettes contained the low-affinity calcium-sensitive fluorescent dye Oregon green 488 BAPTA-5 N. Depolarization of the Purkinje cell elicited an increase in the intracellular calcium concentration (Fig. 5Aa); in the same neuron, the depolarization suppressed sIPSCs to 71% of PRE (Fig. 5Ab). Depolarization applied in the same neuron in the presence of orlistat (10−5m) elicited a calcium concentration increase that was slightly greater than the increase before orlistat (Fig. 5Ba); however, sIPSCs were no longer suppressed (Fig. 5Bb). The statistical evaluation confirms these observations. The depolarization-induced rise in intracellular calcium concentration increased slightly in the presence of orlistat (Fig. 5C). At the same time, however, the depolarization caused only a small suppression of sIPSCs (Fig. 5D). Thus, orlistat does not block DSI by inhibiting calcium influx into the Purkinje cells. Orlistat blocked suppression of sIPSCs also if this was triggered by calcium released by flash pulses from the calcium caging compound DM-nitrophen (M.J. Urbanski & B. Szabo, unpublished observations). As orlistat did not inhibit calcium influx and it prevented sIPSC suppression elicited by calcium uncaging, it is very likely that orlistat blocked DSI acting downstream of calcium entry into the postsynaptic Purkinje cell, most probably by inhibiting diacylglycerol lipase.
Figure 5. Orlistat attenuates DSI without inhibiting the depolarization-induced increase in intracellular calcium concentration in Purkinje cells.
Aa, nine depolarizing pulses (from −70 mV to 0 mV for 100 ms) at 1 Hz elicited an increase in calcium concentration in Purkinje cells. The fluorescence images were obtained before (1) and during stimulation (2) and a differential image was calculated (the scale coding of fluorescence intensities in the differential image differs from the scale coding of intensities in the images obtained before and during stimulation). Ab, the depolarization suppressed the cumulative amplitude of sIPSCs to 71% of PRE. Ba and Bb, in the same neuron, in the presence of orlistat, the depolarization led to a calcium concentration increase, but the cumulative amplitude of sIPSCs (108% of PRE) was not changed. C, statistical evaluation of the effects of solvent and orlistat on the calcium concentration increase during depolarization. Solvent and orlistat were superfused as indicated. ΔF/F0 values were expressed as percentages of the initial reference value PRE. Cadmium superfusion at the end of the experiments abolished the depolarization-induced increases in calcium concentration. Means ± s.e.m. of nine (solvent) and eight (orlistat) experiments. D, in the same experiments, depolarization suppressed sIPSCs in the presence of solvent, but not in the presence of orlistat. Means ± s.e.m. of 14 (solvent) and 11 (orlistat) experiments. The filled symbols (C) and the filled column (D) indicate significant difference versus PRE and asterisk indicates significant difference versus solvent (P < 0.05).
Involvement of calmodulin and Ca2+–calmodulin-dependent protein kinase II in DSI
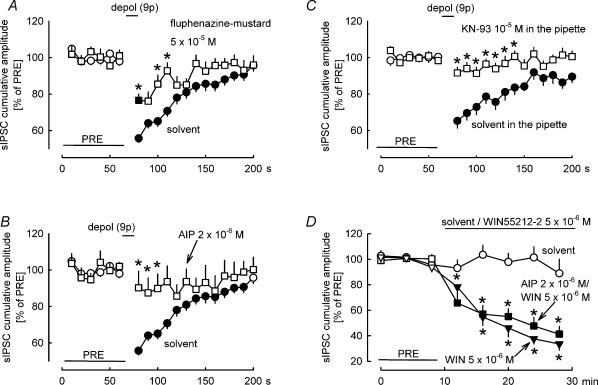
As mentioned above, endocannabinoid synthesis during DSI is triggered by increased calcium levels in postsynaptic neurons. How will enhanced calcium levels lead to 2-AG synthesis? Our hypothesis was that calmodulin and Ca2+–calmodulin-dependent protein kinase II are involved in the transmission chain leading to 2-AG synthesis. Therefore, at first, we studied the effects of the calmodulin inhibitor fluphenazine-mustard (Hait et al. 1987) on DSI. DSI was attenuated in the presence of fluphenazine-mustard (5 × 10−5m) (Fig. 6A). In the second step, the effects of the Ca2+–calmodulin-dependent protein kinase II inhibitors autocamtide-2-related inhibitory peptide (Ishida & Fujisawa, 1995) and KN-93 (Takao et al. 2005; Rezazadeh et al. 2006) were tested. Autocamtide-2-related inhibitory peptide (2 × 10−6m) abolished DSI (Fig. 6B). One might argue that the compund interfered with the effect of the released endocannabinoid on the presynaptic axon terminal. In order to rule out this possibility, we studied the interaction between the synthetic cannabinoid agonist WIN55212-2 and autocamtide-2 related inhibitory peptide on the cumulative amplitude of sIPSCs (Fig. 6D). The suppression of the cumulative amplitude of sIPSCs by WIN55212-2 (5 × 10−6m) was not affected by autocamtide-2 related inhibitory peptide (2 × 10−6m) (Fig. 6D). KN-93 (10−5m), applied with the intracellular solution, also abolished DSI (Fig. 6C). This latter experiment confirms the role of Ca2+–calmodulin-dependent protein kinase II in DSI and localizes the action of the enzyme to the postsynaptic Purkinje cell.
Figure 6. Effects of inhibition of calmodulin and Ca2+–calmodulin-dependent protein kinase II on DSI.
A–C, DSI was elicited by nine depolarizing pulses (from −70 to 0 mV for 100 ms) at 1 Hz. A, DSI was attenuated in the presence of the calmodulin inhibitor fluphenazine-mustard. Means ± s.e.m. of 59 (solvent) and 14 (fluphenazine-mustard) experiments. B, DSI was abolished in the presence of the Ca2+–calmodulin-dependent protein kinase II inhibitor autocamtide-2 related inhibitory peptide (AIP). Means ± s.e.m. of 59 (solvent) and nine (AIP) experiments. C, DSI was abolished in the presence of the Ca2+–calmodulin-dependent protein kinase II inhibitor KN-93 (applied in the patch pipette). Means ± s.e.m. of 21 (solvent) and 22 (KN-93) experiments. D, solvent and the CB1/CB2 cannabinoid receptor agonist WIN55212-2 were superfused as indicated. In one of the WIN55212-2 groups, AIP (2 × 10−6m) was present in the superfusion buffer during the entire experiment. WIN55212-2 inhibited sIPSCs, and this inhibition was not affected by AIP. Means ± s.e.m. of 10 (solvent), 10 (WIN55212-2) and seven (WIN55212-2 in the presence of AIP) experiments. A–D, filled symbol indicates significant difference versus PRE and asterisk indicates significant difference versus solvent (P < 0.05).
Interference with anandamide and 2-AG degradation
As the next procedure in the identification of the endocannabinoid involved in DSI we used drugs that interfere with the degradation of endocannabinoids. Anandamide is uniquely metabolized in the brain by fatty acid amide hydrolase; inhibition of this enzyme always leads to major increases in brain anandamide levels (Di Marzo et al. 1994; Cravatt et al. 2001; Cravatt & Lichtman, 2003). By contrast, it is thought that fatty acid amide hydrolase plays only a minor role in 2-AG degradation; accordingly, inhibition of this enzyme does not result in increases in brain 2-AG levels (Lichtman et al. 2002; but see also Maione et al. 2006). Fatty acid amide hydrolase can be inhibited by URB597 and arachidonoyl-5-hydroxytryptamine (Bisogno et al. 1998; Kathuria et al. 2003). Our hypothesis was that if DSI is mediated by anandamide, then inhibitors of fatty acid amide hydrolase should potentiate DSI.
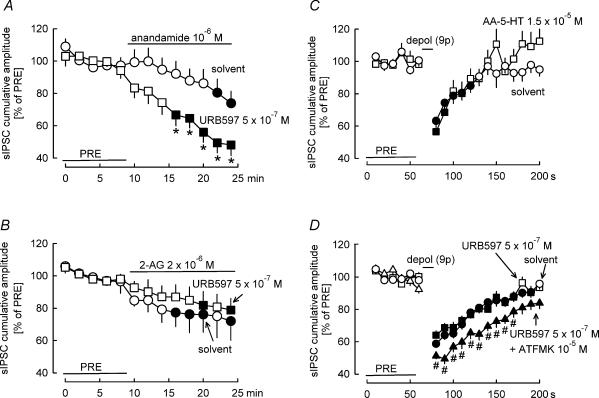
At first we tested the interaction of URB597 with exogenous anandamide and 2-AG. Anandamide was superfused in the presence of solvent or URB597 (Fig. 7A). In solvent-treated slices, anandamide caused a slowly developing and weak inhibition of sIPCSs. In the presence of URB597 (5 × 10−7m), the inhibition by anandamide developed faster and it was also more pronounced. Similar experiments were carried out with 2-AG (Fig. 7B). 2-AG superfused in the presence of solvent caused a small inhibition of sIPSCs. This inhibition was not changed in the presence of URB597 (5 × 10−7m). These observations indicate that under our experimental conditions anandamide degradation, but not 2-AG degradation, is inhibited by URB597 (5 × 10−7m). Concerning the main question of our study, URB597 did not change DSI (Fig. 7D). The other fatty acid amide hydrolase inhibitor, arachidonoyl-5-hydroxytryptamine, also did not affect DSI (Fig. 7C).
Figure 7. Inhibition of fatty acid amide hydrolase does not influence DSI, whereas inhibition of monoglyceride lipase potentiates DSI.
A, superfusion of exogenous anandamide in the presence of solvent elicits a weak inhibition of sIPSCs. In the presence of the fatty acid amide hydrolase inhibitor URB597, the effect of anandamide is potentiated. Means ± s.e.m. of nine (solvent) and 12 (URB597) experiments. B, superfusion of exogenous 2-AG in the presence of solvent elicits a weak inhibition of sIPSCs. The effect of 2-AG is not changed in the presence of URB597. Means ± s.e.m. of 12 (solvent) and nine (URB597) experiments. C and D, DSI was elicited by nine depolarizing pulses (from −70 to 0 mV for 100 ms) at 1 Hz. C, the fatty acid amide hydrolase inhibitor AA-5-HT does not influence DSI. Means ± s.e.m. of 23 (solvent) and 12 (AA-5-HT) experiments. D, the fatty acid amide hydrolase inhibitor URB597 does not change DSI. Additional inhibition of monoglyceride lipase by ATFMK in the presence of URB597 leads to a potentiation of DSI. Means ± s.e.m. of 59 (solvent), 34 (URB597) and 22 (URB597 + ATFMK) experiments. A filled symbol indicates significant difference versus PRE, asterisk indicates significant difference versus solvent and # indicates significant difference versus URB597 (P < 0.05).
2-AG is metabolized in the brain by the enzyme monoglyceride lipase (Dinh et al. 2002; Saario et al. 2004). Our hypothesis was that if DSI is mediated by 2-AG, then inhibitors of monoglyceride lipase should potentiate DSI. Selective inhibitors of monoglyceride lipase have been recently discovered (Makara et al. 2005), but are not yet easily available. Arachidonoyl trifluoromethylketone (ATFMK) inhibits both fatty acid amide hydrolase and monoglyceride lipase (Dinh et al. 2002; Saario et al. 2004). To study the role of monoglyceride lipase in DSI, we observed the effect of ATFMK on DSI in the presence of the fatty acid amide hydrolase inhibitor URB597 (URB597 does not affect monoglyceride lipase; Kathuria et al. 2003). DSI in the combined presence of URB597 (5 × 10−7m) and ATFMK (10−5m) was clearly greater in extent and longer in duration than in the presence of URB597 (5 × 10−7m) alone (Fig. 7D).
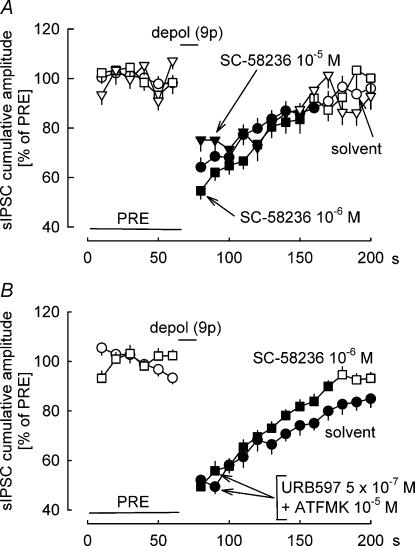
Cyclooxygenase-2 can transform 2-AG to glyceryl prostaglandins (Kozak et al. 2000). Based on the observation that cyclooxygenase-2 inhibitors prolonged DSI in the hippocampus, it was suggested that cyclooxygenase-2 plays an essential role in the degradation of 2-AG released during DSI (Kim & Alger, 2004). We carried out a series of experiments to determine whether cyclooxygenase-2 is involved in the degradation of the endocannabinoid mediating DSI in the cerebellum. The cyclooxygenase-2 inhibitor SC-58236 (10−6 and 10−5m) did not significantly augment or prolong DSI in the cerebellum (Fig. 8A). We reasoned that the role of cyclooxygenase-2 in endocannabinoid metabolism would be more pronounced in the absence of other endocannabinoid-metabolizing pathways. Accordingly, we tested the effects of SC-58236 in brain slices in which fatty acid amide hydrolase and monoglyceride lipase were blocked by URB597 (5 × 10−7m) and ATFMK (10−5m) (Fig. 8B). SC-58236 (10−6m) did not affect DSI also under this condition (Fig. 8B). This suggests that cyclooxygenase-2 does not play a role in the degradation of the endocannabinoid mediating DSI in the cerebellum.
Figure 8. Inhibition of cyclooxygenase-2 does not influence DSI.
DSI was elicited by nine depolarizing pulses (from −70 to 0 mV for 100 ms) at 1 Hz. A, the cyclooxygenase-2 inhibitor SC-58236 (10−6 and 10−5m) does not influence DSI. Means ± s.e.m. of 48 (solvent), 29 (SC-58236 10−6m) and 23 (SC-58236 10−5m) experiments. B, fatty acid amide hydrolase and monoglyceride lipase were inhibited in both groups by URB597 (5 × 10−7m) and ATFMK (10−5m). SC-58236 (10−6m) does not influence DSI under this condition. Means ± s.e.m. of 22 (solvent) and 25 (SC-58236) experiments. A filled symbol indicates significant difference versus PRE (P < 0.05).
Role of 2-AG in DSI and DSE at other synapses
The above observations point to 2-AG as the endocannabinoid mediating DSI at the GABAergic synapses between cerebellar cortical interneurons and Purkinje cells in the mouse. We carried out four final series of experiments to find out whether 2-AG plays a role in DSI and DSE at other synapses.
First, we studied the GABAergic synapses between axons originating in the caudate-putamen (striato-nigral pathway) and substantia nigra pars reticulata neurons in the mouse (Fig. 9A). The striato-nigral pathway was stimulated in the caudate-putamen every 2 s, and eIPSCs were recorded in substantia nigra pars reticulata neurons. After the initial reference period PRE, substantia nigra pars reticulata neurons were depolarized for 5 s. In the presence of solvent, this depolarization suppressed the amplitude of eIPSCs to 75% of PRE (Fig. 9Aa and Ab), similarly as in our previous study (Wallmichrath & Szabo, 2002). The cannabinoid antagonist rimonabant (10−6m; Fig. 9Aa and Ac) as well as the diacylglycerol lipase inhibitor orlistat (10−5m; Fig. 9Aa and Ad) prevented the DSI. This set of observations points to involvement of 2-AG and CB1 receptors also at this synapse.
Figure 9. Effects of orlistat on DSI in different brain regions in the mouse and the rat.
Aa, eIPSCs in mouse substantia nigra pars reticulata neurons were elicited by electrical stimulation of the striato-nigral pathway in the caudate-putamen at 0.5 Hz and three consecutive eIPSCs were averaged. In the presence of solvent, depolarization from −70 to 0 mV for 5 s led to DSI. DSI was abolished in the presence of rimonabant (RIM) and orlistat. Means ± s.e.m. of 14 (solvent), 10 (RIM) and 19 (orlistat) experiments. Ab–Ad, original tracings showing DSI in the presence of solvent and abolishment of DSI by RIM and orlistat. B, eIPSCs in mouse hippocampal CA1 neurons were elicited by electrical stimulation in the vicinity of the recorded neurons at 0.5 Hz and three consecutive eIPSCs were averaged. In the presence of solvent, depolarization from −70 to 0 mV for 5 s led to DSI. DSI was abolished in the presence of RIM, but not changed by orlistat. Means ± s.e.m. of 26 (solvent), 24 (RIM) and 24 (orlistat) experiments. C, sIPSCs were recorded in rat cerebellar cortical Purkinje cells. In the presence of solvent, nine depolarizing pulses (from −70 to 0 mV for 100 ms) at 1 Hz led to DSI. DSI was abolished in the presence of RIM and orlistat. Means ± s.e.m. of 15 (solvent), 10 (RIM) and 17 (orlistat) experiments. A–C, A filled symbol indicates significant difference versus PRE and asterisk indicates significant difference versus solvent (P < 0.05).
Second, we studied GABAergic synapses in the hippocampus (Fig. 9B). CA1 pyramidal neurons were patch clamped and their GABAergic input was stimulated every 2 s with an electrode positioned in the vicinity of the recorded neurons. After the initial reference period PRE, CA1 neurons were depolarized for 5 s. In the presence of solvent, this depolarization suppressed the amplitude of eIPSCs to 76% of PRE (Fig. 9B). The cannabinoid antagonist rimonabant (10−6m) prevented the DSI, pointing to involvement of endocannabinoids and CB1 receptors in this phenomenon. Orlistat (10−5m), however, did not block DSI (Fig. 9B).
Third, we studied the GABAergic synapses between interneurons and Purkinje cells in the cerebellar cortex of the rat (Fig. 9C). The protocol was identical to the protocol used for studying DSI in the mouse cerebellar cortex. In the presence of solvent, depolarization of Purkinje cells by a series of nine pulses led to suppression of the cumulative amplitude of sIPSCs to 50% of PRE (Fig. 9C). In the presence of rimonabant (10−6m) or orlistat (10−5m), DSI no longer occurred (Fig. 9C).
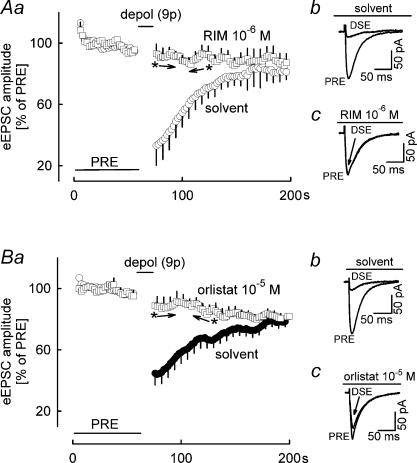
Fourth, we studied the glutamatergic synapses between parallel fibres and Purkinje cells in the cerebellar cortex of the mouse (Fig. 10). Parallel fibres were stimulated every 2 s, and eEPSCs were recorded in Purkinje cells. After the initial reference period PRE, Purkinje cells were depolarized by a series of nine pulses. In the presence of solvent, this depolarization suppressed the amplitude of eEPSCs to 30%–40% of PRE (Fig. 10Aa and 10Ab) (i.e. DSE occurred). In the presence of the cannabinoid antagonist rimonabant (10−6m), DSE was abolished (Fig. 10Aa and 1Ac), thus verifying involvement of endocannabinoids and CB1 receptors. It is important to note that inhibition of diacylglycerol lipase by orlistat (10−5m) fully prevented the DSE (Fig. 10Ba and Bc).
Figure 10. Effects of orlistat on DSE.
eEPSCs in mouse cerebellar cortical Purkinje cells were elicited by electrical stimulation of parallel fibres at 0.5 Hz. A, in the presence of solvent, nine depolarizing pulses (from −70 to 0 mV for 100 ms) at 1 Hz led to DSE. DSE was abolished in the presence of rimonabant (RIM). Means ± s.e.m. of five (solvent) and five (RIM) experiments. For the sake of clarity, only every third s.e.m. bar is displayed. Ab and Ac, original tracings showing DSE in the presence of solvent and abolishment of DSI by RIM. B, DSE was abolished in the presence of orlistat. Means ± s.e.m. of 24 (solvent) and 22 (orlistat) experiments. Bb and Bc, original tracings showing DSE in the presence of solvent and abolishment of DSE by orlistat. A and B, A filled symbol indicates significant difference versus PRE and asterisk indicates significant difference versus solvent (P < 0.05) (between the arrows all points are significantly different).
Discussion
Depolarization-induced suppression of GABAergic inhibition (DSI) has been most frequently studied in the hippocampus and the cerebellar cortex. The present study identifies for the first time 2-AG as the endocannabinoid mediating DSI between interneurons and Purkinje cells in the cerebellar cortex. The identification is based on experiments in which we studied the degradation of the putative endocannabinoids and on experiments in which we studied the steps leading to endocannabinoid synthesis.
Inhibition of the enzyme responsible for degradation of 2-AG, monoglyceride lipase, led to potentiation of DSI in our experiments, pointing to a role of 2-AG. In contrast, a role for anandamide was ruled out by the observation that inhibition of the enzyme responsible for anandamide degradation, fatty acid amide hydrolase, did not enhance DSI. Similar observations led to the identification of 2-AG as the endocannabinoid mediating DSI and DSE in the hippocampus. Thus, DSI in hippocampal slices and DSE in cultured hippocampal neurons was not changed when fatty acid amide hydrolase was inihibited (Kim & Alger, 2004; Makara et al. 2005; Straiker & Mackie, 2005), but was prolonged when monoglyceride lipase was inhibited (Makara et al. 2005). In addition, a role of 2-AG in DSE in cultured hippocampal neurons is supported by the finding that only the kinetics of action of exogenous 2-AG, but not of anandamide and noladin ether, were compatible with the kinetics of DSE (Straiker & Mackie, 2005).
Inhibition of the enzyme responsible for 2-AG production, the sn-1-selective diacylglycerol lipase, by two inhibitors belonging to different chemical classes (RHC80267 and orlistat), led to attenuation and abolishment of DSI. This is the first convincing demonstration of a role of diacylglycerol lipase in DSI. We know only of one study in which a weak inhibition of DSE by RHC80267 was shown, in cultured hippocampal neurons (Straiker & Mackie, 2005). As shown by the data in Table 1, orlistat selectively inhibits diacylglycerol lipase: it does not interfere with 2-AG degradation, anandamide production and degradation and with cannabinoid receptors. In our brain slice experiments it did not activate or block CB1 receptors and it inhibited DSI at a step downstream of calcium entry into the postsynaptic Purkinje cells. Therefore, it is very likely that orlistat (and RHC80267) blocked DSI by inhibiting endocannabinoid production at the level of diacylglycerol lipase.
Consistent with a role of 2-AG in retrograde signalling, all necessary molecular components are present in the appropriate compartments at the synapses between interneurons and Purkinje cells. The enzyme producing 2-AG, diacylglycerol lipase, is highly expressed in the dendrites of Purkinje cells (Bisogno et al. 2003; Yoshida et al. 2006). The target receptors, CB1 receptors, are present in the presynaptic axon terminals of interneurons (Diana et al. 2002; Szabo et al. 2004; Kawamura et al. 2006). Finally, the enzyme degrading 2-AG, monoglyceride lipase, is present in axon terminals in the molecular layer (Dinh et al. 2002; Gulyas et al. 2004; Saario et al. 2004). The anandamide degrading enzyme fatty acid amide hydrolase is localized in Purkinje cell dendrites (Gulyas et al. 2004); this localization would be disadvantageous if retrograde signalling was to be mediated by anandamide. Consistent with the role of 2-AG in retrograde signalling is its high concentration in the brain (much higher than that of anandamide; Sugiura et al. 1995; Stella et al. 1997; Jung et al. 2005). Moreover, electrical stimulation of neurons leads to an increase in 2-AG biosynthesis, but not of anandamide biosynthesis (in the hippocampus; Stella et al. 1997).
The results of our experiments with orlistat suggest that DSE in the mouse cerebellum, DSI in the mouse substantia nigra pars reticulata and DSI in the rat cerebellum are also mediated by 2-AG. As shown by others, DSI and DSE in the hippocampus are also mediated by 2-AG (Kim & Alger, 2004; Makara et al. 2005; Straiker & Mackie, 2005).
Several observations indicate that 2-AG is the endocannabinoid released during short-term retrograde signalling triggered by stimulation of postsynaptic Gαq/11 protein-coupled receptors. Thus, inhibition of GABAergic transmission elicited by stimulation of type 1 metabotropic glutamate receptors on cerebellar Purkinje cells was attenuated by the phospholipase C inhibitor U73122 and by RHC80267 (Galante & Diana, 2004). Activity-dependent short suppression of excitatory transmission in the cerebellar cortex and the ventral tegmental area was also inhibited by orlistat and RHC80267 (Melis et al. 2004; Safo & Regehr, 2005).
There are some recent observations on the endocannabinoid involved in long-term synaptic plasticity. Long-term depression in the cerebellum and hippocampus was abolished in the presence of RHC80267 and orlistat (Chevaleyre & Castillo, 2003; Safo & Regehr, 2005). These findings are compatible with a role of 2-AG. However, long-term depression in the amygdala was stronger in mice lacking fatty acid amide hydrolase and not changed by U73122 and RHC80267 – findings pointing to a role of anandamide (Azad et al. 2004).
Together, the experiments studying the chemical nature of the endocannabinoids involved in retrograde signalling suggest that 2-AG is involved in DSI and DSE, short-term retrograde signalling driven by activation of Gαq/11 protein-coupled receptors and long-term synaptic plasticity at the majority of synapses. Yet, the role of anandamide should not be underestimated. For example, experiments in which the biosynthetic pathway of ananamide is manipulated may uncover hitherto unknown roles for anandamide in synaptic plasticity.
It is remarkable that pathways of 2-AG production may differ depending on the induction protocol. The operation of phospholipase C is necessary for long-term depression and for retrograde signalling elicited by stimulation of Gαq/11 protein-coupled receptors, but not for DSI and DSE (Chevaleyre & Castillo, 2003; Hashimotodani et al. 2005; Maejima et al. 2005; Brenowitz et al. 2006; Edwards et al. 2006). The phospholipase C inhibitor U73122 did not change DSI in our experiments, showing that phospholipase C is also not necessary for DSI in the mouse cerebellar cortex. It has been shown that in neurons, diacylglycerol for 2-AG production can be generated not only by phospholipase C, but also via other pathways (Bisogno et al. 1999; Sugiura et al. 2002).
Less clear is the role of diacylglycerol lipase. In the hippocampus, retrograde signalling elicited by stimulation of muscarinic acetylcholine receptors and long-term depression were more sensitive than DSI to inhibitors of diacylglycerol lipase (Chevaleyre & Castillo, 2003; Edwards et al. 2006). Our own experiments verified the resistance of hippocampal DSI against the diacylglycerol lipase inhibitor orlistat. Similar observations have been made in the cerebellum by Safo & Regehr (2005). Thus, short-term retrograde signalling elicited by stimulation of parallel fibres and long-term depression elicited by combined stimulation of parallel fibres and climbing fibres was inhibited by the diacylglycerol lipase inhibitors RHC80267 and orlistat. In contrast, DSE and DSI were not blocked by RHC80267 and orlistat (Safo & Regehr, 2005; Brenowitz et al. 2006; the reason for the obvious discrepancy between these findings and our findings is not known). Thus, it seems that diacylglycerol lipase participates in the endo-cannabinoid production in retrograde signalling elicited by stimulation of Gαq/11 protein-coupled receptors and in some forms of long-term depression. According to our own results, DSI and DSE can be mediated by 2-AG produced by diacylglycerol lipase. However, it seems that under certain conditions, DSI and DSE is mediated by 2-AG which is not produced by diacylglycerol lipase.
It is not known how elevated intracellular calcium levels lead to enhanced endocannabinoid production in the postsynaptic neuron during DSI and DSE. The role of calmodulin and Ca2+–calmodulin-dependent protein kinase II in long-term synaptic plasticity is well recognized (Kano et al. 1996; Fink & Meyer, 2002; Xia & Storm, 2005). Our results suggest for the first time that these two calcium-dependent messengers are essential for endocannabinoid production during short-term synaptic plasticity such as DSI. Calmodulin and Ca2+–calmodulin-dependent protein kinase II (a group of kinases) are present in cerebellar Purkinje cells and are activated by depolarization protocols similar to that used in the present study (see Kano et al. 1996). Theoretically, the activity of both phospholipase C and diacylglycerol lipase might be stimulated during calcium-induced endocannabinoid release. As phospholipase C is not necessary for DSI, it is likely that the target of calmodulin and Ca2+–calmodulin-dependent protein kinase II during DSI is diacylglycerol lipase. However, further experiments are necessary to characterize exactly the calcium-dependent signalling cascade operating in the postsynaptic cell during DSI and DSE.
A corollary observation of the present study is that cyclooxygenase-2 does not participate in 2-AG degradation in the cerebellum. We employed SC-58236, a potent and selective inhibitor of cyclooxygenase-2 (IC50 for inhibition of cyclooxygenase-2, 10−8m; IC50 for inhibition of cyclooxygenase-1, 1.8 × 10−5m; Penning et al. 1997). It is very likely that at concentrations 100- to 1000-fold higher than its IC50 value for inhibition of cyclooxygenase-2 in cell free systems, SC-58236 indeed inhibited cyclooxygenase-2 in our cerebellar slices. Yet, the magnitude and duration of DSI were not changed, even when other 2-AG degrading pathways were inhibited. Thus, it is probable that in the cerebellum, 2-AG is solely metabolized by monoglyceride lipase, as suggested also by previous biochemical experiments (Saario et al. 2004). Notably, the recent study of Straiker & Mackie (2005) also showed that cyclooxygenase-2 does not play a role in the metabolism of 2-AG during hippocampal DSE.
Activity-dependent alteration of the strength of synaptic transmission – synaptic plasticity – is a widely occurring phenomenon and is thought to be the basis of memory formation and learning. Endocannabinoids mediate short- and long-term depression of synaptic strength in many brain regions (Alger, 2002; Wilson & Nicoll, 2002; Freund et al. 2003; Gerdeman & Lovinger, 2003; Diana & Marty, 2004; Chevaleyre et al. 2006). Importantly, endocannabinoid signalling can be activated by physiological and pathophysiological stimuli (e.g. Robbe et al. 2002; Brown et al. 2003; Chen et al. 2003; Melis et al. 2004; Brenowitz & Regehr, 2005; Maejima et al. 2005; Brenowitz et al. 2006). The identification of the endocannabinoid, and of the steps leading to its synthesis, involved in one form of short-term depression is an important advance in the understanding of these endocannabinoid-mediated processes. Developing drugs that interfere with the synthesis and degradation of 2-AG will make it possible to influence synaptic plasticity and its behavioural correlates.
Acknowledgments
This study was supported by the Deutsche Forschung-sgemeinschaft (Bela Szabo; Sz 72/5−2) and the Volkswagenstiftung (Vincenzo Di Marzo.).
References
- Alger BE. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol. 2002;68:247–286. doi: 10.1016/s0301-0082(02)00080-1. [DOI] [PubMed] [Google Scholar]
- Appendino G, de Petrocellis L, Trevisani M, Minassi A, Daddario N, Moriello AS, et al. Development of the first ultra-potent ‘capsaicinoid’ agonist at transient receptor potential vanilloid type 1 (TRPV1) channels and its therapeutic potential. J Pharmacol Exp Ther. 2005;312:561–570. doi: 10.1124/jpet.104.074864. [DOI] [PubMed] [Google Scholar]
- Azad SC, Monory K, Marsicano G, Cravatt BF, Lutz B, Zieglgänsberger W, Rammes G. Circuitry for associative plasticity in the amygdala involves endocannabinoid signaling. J Neurosci. 2004;24:9953–9961. doi: 10.1523/JNEUROSCI.2134-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Cascio MG, Saha B, Mahadevan A, Urbani P, Minassi A, et al. Development of the first potent and specific inhibitors of endocannabinoid biosynthesis. Biochim Biophys Acta. 2006;1761:205–212. doi: 10.1016/j.bbalip.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Melck D, De Petrocellis L, Bobrov MY, Gretskaya NM, Bezuglov VV, Sitachitta N, Gerwick WH, Di Marzo V. Arachidonoylsrotonin and other novel inhibitors of fatty acid amide hydrolase. Biochem Biophys Res Commun. 1998;248:515–522. doi: 10.1006/bbrc.1998.8874. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Melck D, De Petrocellis L, Di Marzo V. Phosphatidic acid as the biosynthetic precursor of the endocannabinoid 2-arachidonoylglycerol in intact mouse neuroblastoma cells stimulated with ionomycin. J Neurochem. 1999;72:2113–2119. doi: 10.1046/j.1471-4159.1999.0722113.x. [DOI] [PubMed] [Google Scholar]
- Brenowitz SD, Best AR, Regehr WG. Sustained elevation of dendritic calcium evokes widespread endocannabinoid release and suppression of synapses onto cerebellar Purkinje cells. J Neurosci. 2006;26:6841–6850. doi: 10.1523/JNEUROSCI.1280-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz SD, Regehr WG. Calcium dependence of retrograde inhibition by endocannabinoids at synapses onto Purkinje cells. J Neurosci. 2003;23:6373–6384. doi: 10.1523/JNEUROSCI.23-15-06373.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz SD, Regehr WG. Associative short-term synaptic plasticity mediated by endocannabinoids. Neuron. 2005;45:419–431. doi: 10.1016/j.neuron.2004.12.045. [DOI] [PubMed] [Google Scholar]
- Brown SP, Brenowitz SD, Regehr WG. Brief presynaptic bursts evoke synapse-specific retrograde inhibition mediated by endogenous cannabinoids. Nat Neurosci. 2003;6:1048–1057. doi: 10.1038/nn1126. [DOI] [PubMed] [Google Scholar]
- Brown SP, Safo PK, Regehr WG. Endocannabinoids inhibit transmission at granule cell to Purkinje cell synapses by modulating three types of presynaptic calcium channels. J Neurosci. 2004;24:5623–5631. doi: 10.1523/JNEUROSCI.0918-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio MG, Minassi A, Ligresti A, Appendino G, Burstein S, Di Marzo V. A structure-activity relationship study on N-arachidonoyl-amino acids as possible endogenous inhibitors of fatty acid amide hydrolase. Biochem Biophys Res Commun. 2004;314:192–196. doi: 10.1016/j.bbrc.2003.12.075. [DOI] [PubMed] [Google Scholar]
- Chen K, Ratzliff A, Hilgenberg L, Gulyas A, Freund TF, Smith M, Dinh TP, Piomelli D, Mackie K, Soltesz I. Long-term plasticity of endocannabinoid signaling induced by developmental seizures. Neuron. 2003;39:599–611. doi: 10.1016/s0896-6273(03)00499-9. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–472. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–75. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A. 2001;98:9371–9476. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Lichtman AH. Fatty acid amide hydrolase: an emerging target in the endocannabinoid system. Curr Opin Chem Biol. 2003;7:469–475. doi: 10.1016/s1367-5931(03)00079-6. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. The biosynthesis, fate and pharmacological properties of endocannabinoids. In: Pertwee RG, editor. Cannabinoids, Handbook of Experimental Pharmacology. Vol. 168. Heidelberg: Springer-Verlag; 2005. pp. 147–185. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz J-C, Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- Diana MA, Levenes C, Mackie K, Marty A. Short-term retrograde inhibition of GABAergic synaptic currents in rat Purkinje cells is mediated by endogenous cannabinoids. J Neurosci. 2002;22:200–208. doi: 10.1523/JNEUROSCI.22-01-00200.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana MA, Marty A. Characterization of depolarization-induced suppression of inhibition using paired interneuron – Purkinje cell recordings. J Neurosci. 2003;23:5906–5918. doi: 10.1523/JNEUROSCI.23-13-05906.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana MA, Marty A. Endocannabinoid-mediated short-term synaptic plasticity: depolarization-induced suppression of inhibition (DSI) and depolarization-induced suppression of excitation (DSE) Br J Pharmacol. 2004;142:9–19. doi: 10.1038/sj.bjp.0705726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DA, Kim J, Alger BE. Multiple mechanisms of endocannabinoid response initiation in hippocampus. J Neurophysiol. 2006;95:67–75. doi: 10.1152/jn.00813.2005. [DOI] [PubMed] [Google Scholar]
- Engler B, Freiman I, Urbanski M, Szabo B. Effects of exogenous and endogenous cannabinoids on GABAergic neurotransmission between the caudate-putamen and the globus pallidus in the mouse. J Pharmacol Exp Ther. 2006;316:608–617. doi: 10.1124/jpet.105.092718. [DOI] [PubMed] [Google Scholar]
- Fink CC, Meyer T. Molecular mechanisms of CaMKII activation in neuronal plasticity. Curr Opin Neurobiol. 2002;12:293–299. doi: 10.1016/s0959-4388(02)00327-6. [DOI] [PubMed] [Google Scholar]
- Freiman I, Anton A, Monyer H, Urbanski MJ, Szabo B. Analysis of the effects of cannabinoids on identified synaptic connections in the caudate-putamen by paired recordings in transgenic mice. J Physiol. 2006;575:789–806. doi: 10.1113/jphysiol.2006.114272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Galante M, Diana MA. Group I metabotropic glutamate receptors inhibit GABA release at interneuron – Purkinje cell synapses through endocannabinoid production. J Neurosci. 2004;24:4865–4874. doi: 10.1523/JNEUROSCI.0403-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman GL, Lovinger DM. Emerging roles for endocannabinoids in long-term synaptic plasticity. Br J Pharmacol. 2003;140:781–789. doi: 10.1038/sj.bjp.0705466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Gulyas AI, Cravatt BF, Bracey MH, Dinh TP, Piomelli D, Boscia F, Freund TF. Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci. 2004;20:441–458. doi: 10.1111/j.1460-9568.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- Hait WN, Glazer L, Kaiser C, Cross J, Kennedy KA. Pharmacological properties of fluphenazine-mustard, an irreversible calmodulin antagonist. Mol Pharmacol. 1987;32:404–409. [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Tsubokawa H, Ogata H, Emoto K, Maejima T, Araishi K, Shin H-S, Kano M. Phospholipase Cβ serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron. 2005;45:257–268. doi: 10.1016/j.neuron.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Ishida A, Fujisawa H. Stabilization of calmodulin-dependent protein kinase II through the autoinhibitory domain. J Biol Chem. 1995;270:2163–2170. doi: 10.1074/jbc.270.5.2163. [DOI] [PubMed] [Google Scholar]
- Jung K-M, Mangieri R, Stapleton C, Kim J, Fegley D, Wallace M, Mackie K, Piomelli D. Stimulation of endocannabinoid formation in brain slice cultures through activation of group I metabotropic glutamate receptors. Mol Pharmacol. 2005;68:1196–1202. doi: 10.1124/mol.105.013961. [DOI] [PubMed] [Google Scholar]
- Kano M, Kano M, Fukunaga K, Konnerth A. Ca2+-induced rebound potentiation of γ-aminobutyric acid-mediated currents requires activation of Ca2+/calmodulin-dependent kinase II. Proc Natl Acad Sci U S A. 1996;93:13351–13356. doi: 10.1073/pnas.93.23.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, Ohno-Shosaku T, Kano M. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci. 2006;26:2991–3001. doi: 10.1523/JNEUROSCI.4872-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Alger BE. Inhibition of cyclooxygenase-2 potentiates retrograde endocannabinoid effects in hippocampus. Nat Neurosci. 2004;7:697–698. doi: 10.1038/nn1262. [DOI] [PubMed] [Google Scholar]
- Kim J, Isokawa M, Ledent C, Alger BE. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neurosci. 2002;22:10182–10191. doi: 10.1523/JNEUROSCI.22-23-10182.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak KR, Rowlinson SW, Marnett LJ. Oxygenation of the endocannabinoid, 2-arachidonoylglycerol, to glyceryl prostaglandins by cyclooxygenase-2. J Biol Chem. 2000;275:33744–33749. doi: 10.1074/jbc.M007088200. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Carter AG, Regehr WG. Inhibition of interneuron firing extends the spread of endocannabinoid signaling in the cerebellum. Neuron. 2002;34:787–796. doi: 10.1016/s0896-6273(02)00695-5. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Hawkins GE, Griffin G, Cravatt BF. Pharmacological activity of fatty acid amides is regulated, but not mediated, by fatty acid amide hydrolase in vivo. J Pharmacol Exp Ther. 2002;302:73–79. doi: 10.1124/jpet.302.1.73. [DOI] [PubMed] [Google Scholar]
- Llano I, Leresche N, Marty A. Calcium entry increases the sensitivity of cerebellar Purkinje cells to applied GABA and decreases inhibitory synaptic currents. J Neurosci. 1991;6:565–574. doi: 10.1016/0896-6273(91)90059-9. [DOI] [PubMed] [Google Scholar]
- Maejima T, Hashimoto K, Yoshida T, Aiba A, Kano M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron. 2001;31:463–475. doi: 10.1016/s0896-6273(01)00375-0. [DOI] [PubMed] [Google Scholar]
- Maejima T, Oka S, Hashimotodani Y, Ohno-Shosaku T, Aiba A, Wu D, Waku K, Sugiura T, Kano M. Synaptically driven endocannabinoid release requires Ca2+-assisted metabotropic glutamate receptor subtype 1 to phospholipase C β4 signaling cascade in the cerebellum. J Neurosci. 2005;25:6826–6835. doi: 10.1523/JNEUROSCI.0945-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maione S, Bisogno T, de Novellis V, Palazzo E, Cristino L, Valenti M, Petrosino S, Guglielmotti V, Rossi F, Di Marzo V. Elevation of endocannabinoid levels in the ventrolateral periaqueductal grey through inhibition of fatty acid amide hydrolase affects descending nociceptive pathways via both cannabinoid receptor type 1 and transient receptor potential vanilloid type-1 receptors. J Pharmacol Exp Ther. 2006;316:969–982. doi: 10.1124/jpet.105.093286. [DOI] [PubMed] [Google Scholar]
- Makara JK, Mor M, Fegley D, Szabo SI, Kathuria S, Astarita G, Duranti A, Tontini A, Tarzia G, Rivara S, Freund TF, Piomelli D. Selective inhibition of 2-AG hydrolysis enhances endocannabinoid signaling in hippocampus. Nat Neurosci. 2005;8:1139–1141. doi: 10.1038/nn1521. [DOI] [PubMed] [Google Scholar]
- Marcaggi P, Attwell D. Endocannabinoid signaling depends on the spatial pattern of synapse activation. Nat Neurosci. 2005;8:776–781. doi: 10.1038/nn1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Fride E, Di Marzo V. Endocannabinoids. Eur J Pharmacol. 1998;359:1–18. doi: 10.1016/s0014-2999(98)00649-9. [DOI] [PubMed] [Google Scholar]
- Melis M, Perra S, Muntoni AL, Pillolla G, Lutz B, Marsicano G, Di Marzo V, Gessa GL, Pistis M. Prefrontal cortex stimulation induces 2-arachidonoyl-glycerol-mediated suppression of excitation in dopamine neurons. J Neurosci. 2004;24:10707–10715. doi: 10.1523/JNEUROSCI.3502-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama T, Urade R, Kito M. Purification and characterization of diacylglycerol lipase from human platelets. J Biochem. 1999;125:1077–1085. doi: 10.1093/oxfordjournals.jbchem.a022389. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Tsubokawa H, Mizushima I, Yoneda N, Zimmer A, Kano M. Presynaptic cannabinoid sensitivity is a major determinant of depolarization-induced retrograde suppression at hippocampal synapses. J Neurosci. 2002;22:3864–3872. doi: 10.1523/JNEUROSCI.22-10-03864.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem. 2004;279:5298–5305. doi: 10.1074/jbc.M306642200. [DOI] [PubMed] [Google Scholar]
- Penning TD, Talley JJ, Bertenshaw SR, Carter JS, Collins PW, Docter S, et al. Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl) -1H-pyrazol-1-yl]benze nesulfonamide (SC-58635, celecoxib) J Med Chem. 1997;40:1347–1365. doi: 10.1021/jm960803q. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacological actions of cannabinoids. In: Pertwee RG, editor. Cannabinoids, Handbook of Experimental Pharmacology. Vol. 168. Heidelberg: Springer-Verlag; 2005. pp. 1–51. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Rezazadeh S, Claydon T, Fedida D. KN-93 (2-[N-(2-hydroxyethyl)]-N-(4-methoxybenzenesulfonyl)]-amino-N-(4-chlorocinnamyl)-N-methylbenzylamine), a calcium/calmodulin-dependent protein kinase II inhibitor, is a direct extracellular blocker of voltage-gated potassium channels. J Pharmacol Exp Ther. 2006;317:292–299. doi: 10.1124/jpet.105.097618. [DOI] [PubMed] [Google Scholar]
- Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci U S A. 2002;99:8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saario SM, Savinainen JR, Laitinen JT, Järvinen T, Niemi R. Monoglyceride lipase-like enzymatic activity is responsible for hydrolysis of 2-arachidonoylglycerol in rat cerebellar membranes. Biochem Pharmacol. 2004;67:1381–1387. doi: 10.1016/j.bcp.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Safo PK, Regehr WG. Endocannabinoids control the induction of cerebellar LTD. Neuron. 2005;48:647–659. doi: 10.1016/j.neuron.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- Straiker A, Mackie K. Depolarization-induced suppression of excitation in murine autaptic hippocampal neurones. J Physiol. 2005;569:501–517. doi: 10.1113/jphysiol.2005.091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Kobayashi Y, Oka S, Waku K. Biosynthesis and degradation of anandamide and 2-arachidonoylglycerol and their possible physiological significance. Prostaglandins Leukot Essent Fatty Acids. 2002;66:173–192. doi: 10.1054/plef.2001.0356. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in the brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- Szabo B, Schlicker E. Effects of cannabinoids on neurotransmission. In: Pertwee RG, editor. Cannabinoids, Handbook of Experimental Pharmacology. Vol. 168. Heidelberg: Springer-Verlag; 2005. pp. 372–365. [DOI] [PubMed] [Google Scholar]
- Szabo B, Than M, Thorn D, Wallmichrath I. Analysis of the effects of cannabinoids on synaptic transmission between basket and Purkinje cells in the cerebellar cortex of the rat. J Pharmacol Exp Ther. 2004;310:915–925. doi: 10.1124/jpet.104.066670. [DOI] [PubMed] [Google Scholar]
- Szabo B, Urbanski MJ, Di Marzo V. The role of 2-arachidonylglycerol in retrograde signaling. Symposium on the Cannabinoids, International Cannabinoid Research Society, Burlington, Vermont, S14. 2005 [Google Scholar]
- Takahashi KA, Linden DJ. Cannabinoid receptor modulation of synapses received by cerebellar Purkinje cells. J Neurophysiol. 2000;83:1167–1180. doi: 10.1152/jn.2000.83.3.1167. [DOI] [PubMed] [Google Scholar]
- Takao K, Okamoto K-I, Nakagawa T, Neve RL, Nagai T, Miyawaki A, Hashikawa T, Kobayashi S, Hayashi Y. Visualization of synaptic Ca2+/calmodulin-dependent protein kinase II activity in living neurons. J Neurosci. 2005;25:3107–3112. doi: 10.1523/JNEUROSCI.0085-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanski MJ, Mendiguren A, Urbani P, Di Marzo V, Szabo B. Depolarisation-induced suppression of inhibition is mediated by 2-arachidonoylglycerol. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:R72. [Google Scholar]
- Varma N, Carlson GC, Ledent C, Alger BE. Metabotropic glutamate receptors drive the endocannabinoid system in hippocampus. J Neurosci. 2001;21:RC188. doi: 10.1523/JNEUROSCI.21-24-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallmichrath I, Szabo B. Cannabinoids inhibit striatonigral GABAergic neurotransmission in the mouse. Neuroscience. 2002;113:671–682. doi: 10.1016/s0306-4522(02)00109-4. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- Xia Z, Storm DR. The role of calmodulin as a signal integrator for synaptic plasticity. Nat Rev Neurosci. 2005;6:267–276. doi: 10.1038/nrn1647. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Fukaya M, Uchigashima M, Miura E, Kamiya H, Kano M, Watanabe M. Localization of diacylglycerol lipase-α around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J Neurosci. 2006;26:4740–4751. doi: 10.1523/JNEUROSCI.0054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]