Abstract
Electrophysiological recordings of propagated compound action potentials (CAPs) and axonal Ca2+ measurements using confocal microscopy were used to study the interplay between AMPA receptors and intracellullar Ca2+ stores in rat spinal dorsal columns subjected to in vitro combined oxygen and glucose deprivation (OGD). Removal of Ca2+ or Na+ from the perfusate was protective after 30 but not 60 min of OGD. TTX was ineffective with either exposure, consistent with its modest effect on ischaemic depolarization. In contrast, AMPA antagonists were very protective, even after 60 min of OGD where 0Ca2+ + EGTA perfusate was ineffective. Similarly, blocking ryanodine receptor-mediated Ca2+ mobilization from internal stores (0Ca2+ + nimodipine or 0Ca2+ + ryanodine), or inositol 1,4,5-trisphosphate (IP3)-dependent Ca2+ release (block of group 1 metabotropic glutamate receptors with 1-aminoindan-1,5-dicarboxylic acid, inhibition of phospholipase C with U73122 or IP3 receptor block with 2APB; each in 0Ca2+) were each very protective, with the combination resulting in virtually complete functional recovery after 1 h OGD (97 ± 32% CAP recovery versus 4 ± 6% in artificial cerebrospinal fluid). AMPA induced a rise in Ca2+ concentration in normoxic axons, which was greatly reduced by blocking ryanodine receptors. Our data therefore suggest a novel and surprisingly complex interplay between AMPA receptors and Ca2+ mobilization from intracellular Ca2+ stores. We propose that AMPA receptors may not only allow Ca2+ influx from the extracellular space, but may also significantly influence Ca2+ release from intra-axonal Ca2+ stores. In dorsal column axons, AMPA receptor-dependent mechanisms appear to exert a greater influence than voltage-gated Na+ channels on functional outcome following OGD.
Central nervous system axons have the critical role of transmitting information with high fidelity and reliability between neurons by action potential propagation from the soma to the presynaptic terminals. Axonal disruption often results in serious morbidity, and is a hallmark of a variety of disorders including spinal cord injury, traumatic and ischaemic brain injury and multiple sclerosis. The mechanisms of axo-glial injury are surprisingly complex: in anoxia/ischaemia, for example, a rapid loss of ATP production results in accumulation of axonal Na+ and loss of K+, leading to membrane depolarization and intra-axonal Ca2+ overload (for review see Stys, 2004). Blockade of TTX-sensitive Na+ channels during injury is protective in models of optic nerve anoxia/ischaemia (Stys et al. 1992; Fern et al. 1993; Leppanen & Stys, 1997b; Jiang & Stys, 2000), and in spinal cord trauma (Agrawal & Fehlings, 1996; Teng & Wrathall, 1997) and anoxia (Imaizumi et al. 1997). One of the consequences of intra-axonal Na+ accumulation and depolarization is reversal of Na+-dependent transporters, such as the Na+–Ca2+ exchanger and Na+-dependent glutamate transporters, leading to Ca2+ accumulation, glutamate release and excitotoxic injury, the latter thought to be mediated by ionotropic and metabotropic glutamate receptors (mGluRs). Thus, inhibition of Na+–Ca2+ exchange, glutamate receptors or Na+-dependent glutamate transport is protective against white matter anoxia and trauma (Agrawal & Fehlings, 1997; Wrathall et al. 1997; Li et al. 1999; Rosenberg et al. 1999; Li & Stys, 2000; Tekkok & Goldberg, 2001). Pathological depolarization also activates voltage-sensitive Ca2+ channels; blockade of L-type and N-type channels partially protects against anoxic and traumatic axonal injury (Fern et al. 1995; Imaizumi et al. 1999; Wolf et al. 2001; Ouardouz et al. 2003, 2005). Voltage-gated Ca2+ channels may also promote axonal Ca2+ overload not only by influx of Ca2+ from the extracellular space, but also by triggering release of Ca2+ from internal stores by a mechanism similar to excitation–contraction coupling in muscle (Ouardouz et al. 2003).
Whereas it is now clear that AMPA/kainate receptor blockade is protective in a variety of white matter injury protocols (Agrawal & Fehlings, 1997; Wrathall et al. 1997; Li et al. 1999; Rosenberg et al. 1999; Tekkok & Goldberg, 2001), the precise loci of action of these receptors are not known: glia are likely targets but it is unclear whether axons may respond directly to activation of these receptors. In this study we used an in vitro ischaemic protocol of spinal cord dorsal column injury to study different pathways responsible for inducing axonal damage using both electrophysiological recordings of compound action potentials (CAPs) and confocal microscopy to measure changes in intra-axonal Ca2+ concentration. Our results reveal a surprisingly complex interaction between AMPA receptor-dependent mechanisms and release of Ca2+ from intracellular stores in the induction of white matter ischaemic injury.
Methods
Tissue preparation
All experimental protocols were approved by the institutional animal care committee. Spinal cord dorsal columns were excised from adult Long-Evans male rats (250–350 g) after intracardiac perfusion with cold 0Ca2+ saline solution in animals deeply anaesthetized using intraperitoneal injection of sodium pentobarbital (35 mg kg−1). Depth of anaesthesia was assessed by response to peripheral pain and corneal reflex (for details see Ouardouz et al. 2003). Longitudinal dorsal column slices, measuring approximately 8–10 mm in length × 1 mm thick, were gently dissected from the thoracic spinal cord and placed in an interface recording chamber continuously perfused with artificial cerebrospinal fluid (aCSF) containing (mm): NaCl 126, KCl 3, MgSO4 2, NaHCO3 26, NaH2PO4 1.25, CaCl2 2 and dextrose 10, oxygenated with 95% O2–5% CO2 and gradually warmed to 37°C for 1 h.
Measurements of propagated CAPs
Suction electrodes were used to evoke CAPs by constant-voltage pulses from one end and for recording from the opposite end. Electrodes were only applied for the time required to take readings at the various time points. Electrode resistance was measured and used to correct any instability of the recorded amplitudes generated by the application and removal of the electrodes as previously described (Stys et al. 1991). After recording control CAPs, ischaemia was induced by combined oxygen and glucose deprivation (OGD) for 1 h, by changing to 5% CO2–95% N2 atmosphere in the interface chamber and perfusing with a solution bubbled with 5% CO2–95% N2 containing 10 mm sucrose in place of glucose. CAP recovery after OGD was recorded 1–3 h after reoxygenation and reperfusion with glucose-containing oxygenated aCSF. Drugs were pre-applied 1 h before and continued for 15 min following OGD. Data are presented as means ± s.d.
Resting membrane potential measurements
Compound resting membrane potentials were recorded from dorsal column in vitro using a grease gap chamber at 37°C (for details see Leppanen & Stys, 1997a). The width of the gap was ∼2 mm, and given that glial processes typically extend no more than several hundred microns longitudinally in white matter tracts, axonal potentials could therefore be recorded in isolation. Raw baseline gap potentials (Vg) varied from slice to slice (typical range −20 to −40 mV) because of differences in the short circuit factor (Stämpfli, 1954). Vg recordings in aCSF typically stabilized 90 min after insertion into the gap, and thereafter varied by less than 5% over the next hour of recording. To compare responses over time and between different treatments, ratios of Vg values were calculated at different time points with respect to potentials at time 0 (defined as a stable potential baseline before any experimental treatment). Ischaemia was induced chemically using the glycolytic blocker iodoacetate (1 mm) (Sabri & Ochs, 1971) and the mitochondrial inhibitor NaN3 (2 mm) (Kauppinen & Nicholls, 1986). We found that this method of inducing ischaemia gave more reproducible membrane potential changes than glucose removal and gaseous N2; in these experiments reperfusion was not needed so the rapid but irreversible effects of iodoacetate were acceptable, and even preferred.
Axonal Ca2+ imaging
Freshly excised dorsal columns were loaded with the red dextran-conjugated dye Alexa Fluor 594 as a reference and the dextran-conjugated Ca2+ indicator Oregon Green-488 BAPTA-1 (both from Molecular Probes, Eugene, OR, USA) to monitor Ca2+ fluorescence changes as previously described (Ouardouz et al. 2003). Typically about six different dorsal columns were studied per treatment group. Imaging was done ∼1 mm from the cut ends. As previously demonstrated (Ren et al. 2000; Verbny et al. 2002), loading dyes in this manner results in exclusive staining of axon cylinders allowing identification of axonal regions of interest in isolation. A Nikon C1 Eclipse microscope equipped with a 60 × 1.0 NA water immersion objective was used to monitor fluorescence in confocal mode. Dyes were excited at 488 and 594 nm with Ar and He–Ne lasers, respectively. A dichroic mirror centred at 580 nm split the emission into two channels, which were filtered by 540 ± 30 nm and 630 ± 15 nm bandpass filters. Images were acquired every 60 s at 35°C. Ischaemia was induced chemically as for grease gap experiments using the glycolytic blocker iodoacetate and the mitochondrial inhibitor NaN3; the open configuration of the perfusion chamber mounted on the upright microscope to allow access by the imaging objective made it impossible to adequately maintain a very low O2 tension, hence the choice of chemical inhibitors. Data are presented as a ratio of green Ca2+-dependent signal against the Ca2+-insensitive red channel (see Fig. 6), then percentage change during ischaemia compared to control was calculated individually for each axon segment using ImageTrak software written by PKS (http://www.ohri.ca/stys/imagetrak). In order to control for possible fluorescence changes of intrinsic flavin adenine dinucleotide (FAD) in response to metabolic inhibition, whose excitation/emission overlaps with our Ca2+ indicator, axons were loaded with the red dextran but the green Ca2+ dye was omitted. When dorsal columns were then exposed to identical conditions of chemical ischaemia, a 26% decrease in green fluorescence was observed. However, because absolute FAD fluorescence was about one-third as strong as baseline Oregon Green-488 BAPTA-1 signal, this ischaemic drop in FAD emission would cause a < 10% underestimate in the true rise of the Ca2+ signal; the latter was therefore not corrected for the much smaller FAD fluorescence changes seen with metabolic inhibition.
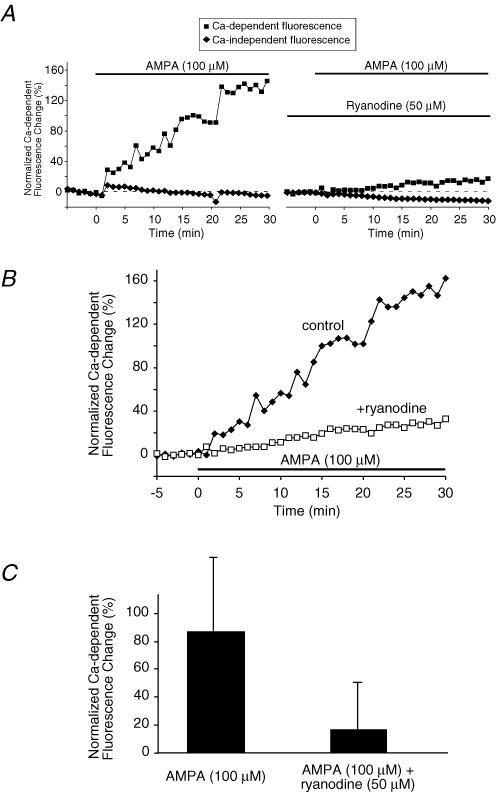
Figure 6. AMPA increases axoplasmic [Ca2+] in spinal cord dorsal column axons measured using confocal microscopy.
A and B, example time courses from one experiment of axoplasmic Ca2+ levels as reported by fluorescence change of Oregon Green-488 BAPTA-1 in normal, non-ischaemic dorsal column axons. AMPA (100 μm; plus 30 μm cyclothiazide to reduce desensitization) induced a substantial Ca2+ rise which was significantly blunted by ryanodine. A, shows Ca2+-dependent (Oregon Green-488 BAPTA-1) and Ca2+-independent (Alexa Fluor 594) fluorescence separately. B, illustrates the ratio of Ca2+-dependent to Ca2+-independent fluorescence to correct for the small downward drift in fluorescence over time. C, bar graph of mean axoplasmic Ca2+ fluorescence changes at 30 min. Fluorescence increased by a mean of 86 ± 53% above baseline after 30 min of AMPA exposure. Addition of ryanodine (50 μm) reduced the AMPA-evoked Ca2+ increase to 16 ± 34% (P < 10−7). These data suggest that AMPA receptors increase axonal [Ca2+] partly by controlling release of Ca2+ from ryanodine receptor-dependent axonal Ca2+ stores (see text). n = 48 and 26 axons for AMPA and AMPA + ryanodine, respectively.
Ca2+ imaging in myelin
Ca2+ changes in the myelin sheath were measured as recently described (Micu et al. 2006). Freshly excised dorsal columns were loaded with the Ca2+ indicator X-rhod-1 to monitor Ca2+ change and with 3,3-dihexyloxacarbocyanine iodide (DiOC6(3)) (500 nm) to outline myelin. Two-photon excited fluorescence images were collected every minute using a custom-modified Nikon D-Eclipse C1 laser-scanning microscope (two-photon excitation was used because confocal imaging results in excessive photobleaching of the X-rhod-1 indicator). Data are presented as a ratio of red Ca2+-dependent signal against the Ca2+-insensitive green channel, then percentage change during AMPA application compared to control was calculated individually for each myelin segment using ImageTrak.
Immunohistochemistry
Deeply anaesthetized adult rats were perfused with saline then 4% paraformaldehyde in 0.1 m phosphate buffer. Dorsal columns were excised, postfixed, treated with methanol for 30 min on ice, and then blocked with 4% normal goat serum in Tris buffer containing 1% Triton X-100 for 1 h. Primary antibodies (GluR1, GluR2/3 and GluR4, Chemicon International, 1:100; NF160, Sigma clone NN18, 1:1000) were applied followed by secondary antibodies (Alexa 488 or Texas Red labelled) at 1:100 dilution. Slides were imaged on a Nikon C1 confocal laser scanning microscope with a 60 × 1.4 NA oil immersion objective.
Statistics
Data are presented as mean ± s.d. and statistical significance was assessed using ANOVA with Dunnett's post hoc test for multiple comparisons with a common control group, or Tukey's one-way ANOVA for multiple comparisons, unless otherwise indicated.
Results
CAP recovery at 30 versus 60 min OGD
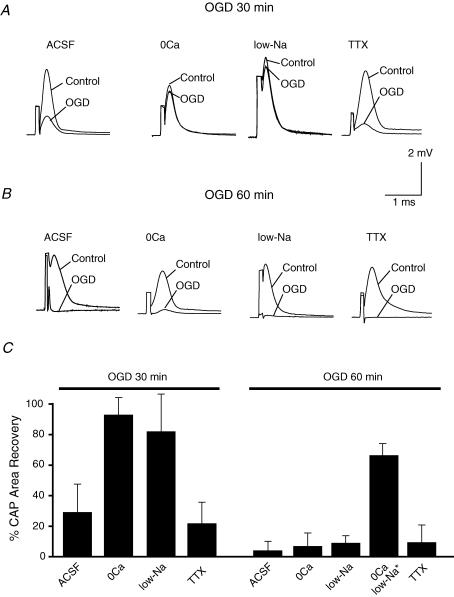
OGD for 30 or 60 min caused severe injury to spinal cord dorsal column axons as assessed by the irreversible decrease of CAP area measured 3 h after the insult, to allow maximal recovery. As shown in Fig. 1, CAPs recovered to 29 ± 19% of control after 30 min OGD (n = 8, Fig. 1A and C) and to 4 ± 6% of control following 60 min of OGD (n = 12, Fig. 1B and C). It is interesting to note that the ability of external 0Ca2+ (with 0.5 mm EGTA) solution to protect dorsal columns against OGD was heavily dependent on duration of the insult: after a 30-min OGD exposure, 0Ca2+ perfusate was completely protective (CAP area recovered to 93 ± 12% of control; n = 6, Fig. 1A and C). In contrast, bath Ca2+ removal was completely ineffective at protecting dorsal columns from 60 min OGD (7 ± 9% of control, n = 8, Fig. 1B and Cversus 4 ± 6% in Ca2+-containing perfusate, P = 1.0). 0Ca2+ perfusate alone, without ischaemia, had no significant effect on CAP magnitude after equivalent washout periods (data not shown). Similarly, reducing bath Na+ to 27 mm by replacing Na+ with the impermeant cation N-methyl-d-glucamine largely rescued dorsal columns from 30 min of OGD (CAP recovery was 82 ± 25% of control, n = 4, Fig. 1A and C) but was again ineffective against 1 h of OGD (9 ± 5% of control, n = 5, Fig. 1B and Cversus 4 ± 6% in normal aCSF, P = 0.13). Only depletion of both bath Ca2+ and Na+ was protective, allowing recovery to 66 ± 9% (Ouardouz et al. 2003). Interestingly, selective blockade of voltage-gated Na+ channels by TTX (0.1 μm) did not protect dorsal columns against either 30 or 60 min OGD (CAP recovery with vs. without TTX, 22 ± 14% vs. 29 ± 19% at 30 min (n = 4, Fig. 1A and C, P = 0.49); 9 ± 12% vs. 4 ± 6% at 1h, (n = 5, Fig. 1B and C, P = 1.0), Exposure to TTX or low Na+ without OGD for 2 h then 3 h wash in normal CSF allowed CAP areas to recover to 79 ± 5% (n = 4) and 97 ± 18% (n = 4) of control, respectively, indicating only modest depression of excitability by these treatments, which would not account for the failure to protect against OGD. These results indicate that both extracellular Na+ and Ca2+ ions contributed critically to dorsal column injury during the first 30 min of OGD; importantly, longer ischaemia recruited additional injury mechanisms that could not be mitigated by removal of external Ca2+ or Na+. Moreover, our observations also indicate that TTX-sensitive voltage-gated Na+ channels are not the only routes by which Na+ gains access into the intracellular compartment during in vitro ischaemia in dorsal columns.
Figure 1. Chemical ischaemia was induced in vitro by switching to N2 atmosphere with glucose replaced by sucrose in the perfusate (oxygen and glucose deprivation, OGD) at 37°C.
A, OGD for 30 min, followed by 3 h of reperfusion caused an irreversible reduction of compound action potential (CAP) area which could be rescued by removal of bath Ca2+ or by low Na+ concentration (29 mm) but not by TTX (0.1 μm) blockade of Na+ channels. B, OGD for 60 min caused a severe injury of spinal cord dorsal column as reflected by a near-complete failure of CAP propagation, which could not be prevented by removal of bath Ca2+ or Na+, nor by addition of TTX. C, bar graph showing quantitative changes in mean CAP area after 30 or 60 min OGD under different experimental conditions. n = 8, 6, 4 and 4 for 30 min OGD for ACSF, 0Ca, low-Na and TTX, respectively, and n = 12, 8, 5, 7 and 5 for 60 min OGD for ACSF, 0Ca, low-Na, 0Ca low-Na and TTX, respectively. *Data from Ouardouz et al. (2003).
Resting membrane potentials in dorsal column axons
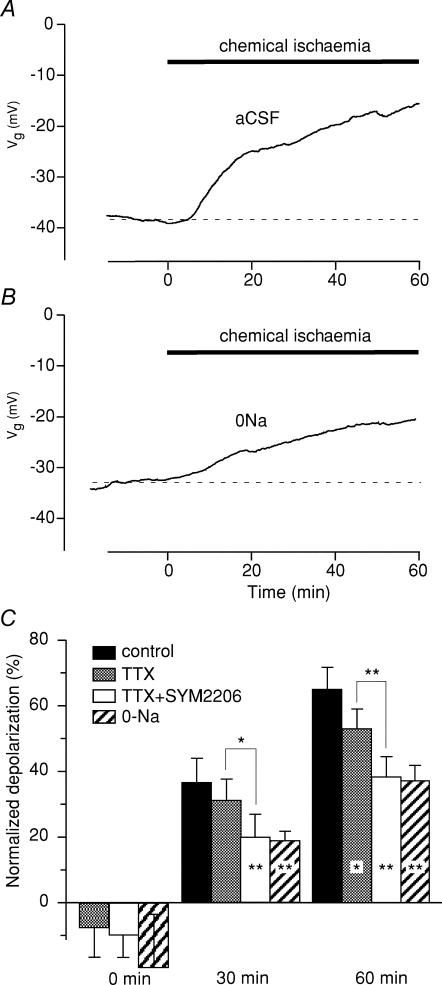
Optic nerve axons exhibit baseline permeability to Na+ that contributes several millivolts of depolarization at rest (Stys et al. 1993; Leppanen & Stys, 1997a; Malek et al. 2003). Dorsal column fibres also displayed a resting Na+ permeability: addition of TTX (prior to chemical ischaemia) shifted resting membrane potential by 7 ± 6% (n = 6, P = 0.009 by paired t test) in the hyperpolarizing direction after 30 min of exposure (Fig. 2C). Addition of the AMPA antagonist SYM2206 (30 μm) did not cause any additional hyperpolarization (10 ± 7%, n = 6, P = 0.27, two-sample t test) indicating that AMPA receptors did not contribute significant ionic permeability in resting axons. During ischaemia, TTX reduced the degree of depolarization (53 ± 6% depolarization at 60 min versus 65 ± 7% without TTX, P = 0.02). The effect was not significant at 30 min, which was even more restrained with the addition of SYM2206 (SYM2206 + TTX versus TTX alone, 20 ± 7% versus 31 ± 7%, P = 0.03 at 30 min; 38 ± 6% and 53 ± 6%, P = 0.003 at 60 min, Fig. 2C). The combination of TTX + SYM2206 had a depolarization-sparing effect identical to Na+-depleted perfusate (38 ± 6% and 37 ± 5% depolarization at 60 min of ischaemia, respectively, P = 0.99).
Figure 2. Effect of chemical ischaemia on the compound resting membrane potential of spinal cord dorsal column axons.
Time 0 denotes application of insult. Zero percentage depolarization denotes no change from normal resting potential, 100% means complete depolarization to 0 mV. A, chemical ischaemia (2 mm NaN3 + 1 mm iodoacetic acid (IAA)) caused a 65 ± 7% depolarization after 60 min of perfusion. B, removal of external Na+ reduced the degree of depolarization. C, summary of different pharmacological manipulations and their effects on ischaemic membrane depolarization. Left group of bars (0 min) shows that all three manipulations caused a significant hyperpolarizing shift in axonal resting potential before ischaemia was applied (see text). Na+-channel blockade (1 μm TTX) was only modestly effective at preventing depolarization during chemical ischaemia (53 ± 6% depolarization at 60 min versus 65 ± 7% without TTX). Combined blockade of Na+ channels and AMPA receptors (TTX + 30 μm SYM2206) reduced ischaemic depolarization more than TTX alone, to a degree comparable to that observed with Na+ removal (TTX + SYM2206, 38 ± 6%; 0Na, 37 ± 5%). *P < 0.05, **P < 0.01. n = 5, 6, 5 and 6 for control, TTX, TTX + SYM2206 and 0Na, respectively.
Role of AMPA receptors during OGD
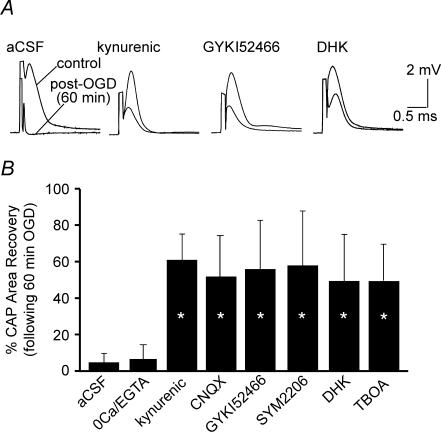
Blockade of AMPA receptors is protective in spinal cord dorsal column anoxia and trauma (Agrawal & Fehlings, 1997; Li et al. 1999). The above results suggest that AMPA receptors were activated during ischaemia, but not at rest. Given the known permeability of some AMPA receptors to Na+ and Ca2+ (Hollmann et al. 1991), we therefore explored whether glutamate receptor antagonists, in addition to reducing ischaemic depolarization, improve CAP recovery after 1 h OGD (Fig. 3), a protocol where neither Ca2+ or Na+ removal were protective (Fig. 1). Kynurenic acid (1–1.5 mm), a broad-spectrum ionotropic glutamate receptor antagonist, afforded highly significant protection against 1 h OGD (CAP recovery 56 ± 27%, n = 6 versus 4 ± 6% without antagonist, P = 1.8 × 10−6). Similar results were obtained using the AMPA/kainate receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 50 μm; 55 ± 24% recovery, n = 6, P = 1.8 × 10−6). The more selective AMPA receptor inhibitors GYKI52466 (100 μm) and SYM2206 (30 μm) (Ouardouz & Durand, 1991; Donevan & Rogawski, 1993; Pei et al. 1999) had similar potency to kynurenic acid and CNQX, with CAP recoveries to 57 ± 30% (n = 5, P = 1.9 × 10−6) and 60 ± 14% (n = 3, P = 6.6 × 10−6) of control, respectively. In addition, blocking glutamate release mediated by reverse Na+-dependent glutamate transport using dihydrokainate or dl-threo-β-benzyloxyaspartic acid (TBOA, 500 μm) were also highly protective (49 ± 28% of control, n = 6, P = 3 × 10−6 and 49.1 ± 19.7% of control n = 5, P = 7 × 10−6, respectively), indicating that endogenous glutamate was released by this mechanism during OGD. Taken together, these results indicate that ischaemia-induced glutamate release activated AMPA receptors to cause injury; of note, these receptors did not mediate injury only by fluxing Ca2+ or Na+ ions, because removal of either was not protective in the 1 h OGD protocol.
Figure 3. Protective effect of glutamate receptors antagonists.
Kynurenic acid (1–1.5 mm), a broad-spectrum glutamate receptor antagonist, CNQX (50 μm, AMPA/kainate receptor antagonist) and the selective AMPA receptor inhibitors GYKI52466 (100 μm) and SYM2206 (30 μm) afforded highly significant protection against 60 min OGD. Blocking glutamate release mediated by reverse Na+-dependent glutamate transport with dihydrokainate (DHK, 1 mm) or TBOA (500 μm) was also highly protective. A, representative CAP tracings. B, bar graph showing quantitative changes in mean CAP area recovery after 60 min OGD under different experimental conditions. *P < 10−4versus aCSF alone. n = 23, 8, 6, 6, 5, 3, 6, 5 respectively, as shown in bar graph.
Intracellular Ca2+ stores during ischaemia
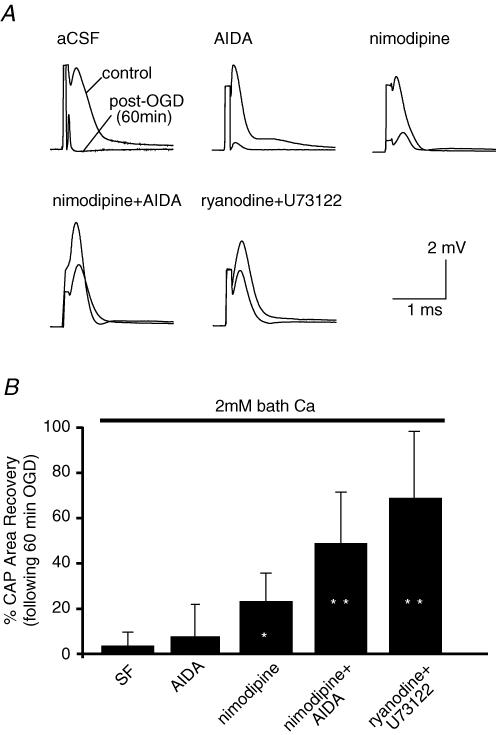
Given the above results, and a report that group 1 mGluR antagonists were protective against dorsal column traumatic injury (Agrawal et al. 1998), we examined whether released glutamate may also signal through mGluRs during OGD. Applied by itself, the group 1 mGluR antagonist 1-aminoindan-1,5-dicarboxylic acid (AIDA, 50 μm) afforded no protection against 1 h OGD (CAP recovered to 8 ± 15% after 1 h OGD/reperfusion, n = 9, Fig. 4). However, one cannot a priori dismiss any potential contribution of group 1 mGluRs (and by inference inositol 1,4,5-trisphosphate (IP3)-dependent Ca2+ stores that are typically controlled by these receptors) because CNS white matter injury depends on a number of parallel pathways of Ca2+ overload (e.g. Na+–Ca2+ exchanger, L-type Ca2+ channels, ryanodine receptors, see Ouardouz et al. 2003, 2005). Similarly, the mean recovery in the presence of nimodipine (10 μm) alone was not statistically different from control (23 ± 13%, n = 6, P = 0.09). In contrast, the combination of the group 1 mGluR inhibitor AIDA together with nimodipine was protective, with mean CAP recovery of 49 ± 23% of control, n = 6, compared to 4 ± 6% in aCSF alone, P = 2.8 × 10−6versus aCSF alone (Fig. 4). Similarly, when Ca2+ release from ryanodine-dependent stores was blocked by ryanodine (30–50 μm) and IP3 synthesis reduced by inhibiting phospholipase C (U73122, 20 μm), CAP recovery was also greatly improved (69 ± 30%, n = 4, P = 1.8 × 10−6versus aCSF alone).
Figure 4. Intracellular Ca2+ stores are mobilized during OGD in spinal cord dorsal columns.
A, group 1 metabotropic glutamate receptor antagonist AIDA (50 μm) afforded no protection against 60 min OGD in normal external Ca2+. Only the combination of AIDA together with nimodipine (10 μm) was protective. Similarly, when Ca2+ release from ryanodine-dependent stores was blocked by ryanodine (50 μm) and IP3 synthesis reduced by inhibiting phospholipase C (U73122, 20 μm), significant protection was seen. B, quantitative summary of CAP area recovery after 3 h reperfusion following 60 min OGD under different experimental conditions. *P < 10−4versus aCSF alone. n = 23, 9, 7, 6 and 4 for aCSF, AIDA, nimodipine, nimodipine + AIDA and ryanodine + U73122, respectively, as shown in bar graph.
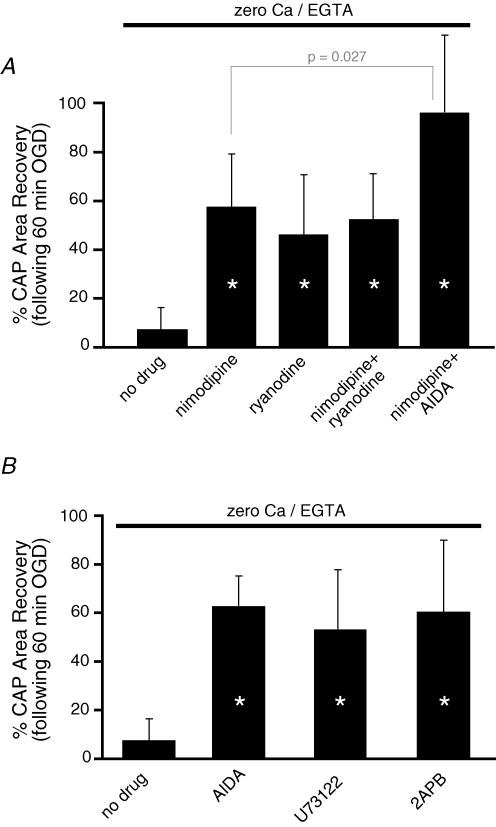
These data suggest that intracellular Ca2+ sources contributed significantly to dorsal column injury. In order to examine the effects of various manipulations on intracellular Ca2+ release, additional studies were performed in perfusate devoid of Ca2+ and with the addition of the Ca2+ chelator EGTA, to remove any influence of Ca2+ entering from the extracellular space. Under these conditions, nimodipine was even more effective than in Ca2+-containing perfusate (CAP area recovered to 57 ± 22% with 0Ca2+ + nimodipine, n = 10, P = 2.5 × 10−4versus 0Ca2+ aCSF, Fig. 5A). The improved protection with nimodipine observed in 0Ca2+ aCSF versus Ca2+-containing aCSF indicates additional parallel Ca2+ influx pathways, such as Na+–Ca2+ exchanger (Ouardouz et al. 2005; for review see Stys, 2004). Similarly, in 0Ca2+ solution, ryanodine or nimodipine alone, or in combination, all conferred roughly the same degree of protection (∼55% CAP area recovery, Fig. 5A), indicating that under these conditions, nimodipine acted mainly to restrain depolarization-dependent Ca2+ release from ryanodine-dependent Ca2+ stores (Ouardouz et al. 2003). The remaining injury may be due to Ca2+ release from ryanodine-independent sources (e.g. IP3 receptor, mitochondria). Interference with IP3-dependent Ca2+ release at multiple points, including (1) group 1 mGluR inhibition (AIDA), (2) reducing IP3 synthesis by blocking phospholipase C (U73122) or (3) IP3 receptor blockade (2-APB) all resulted in nearly identical degrees of postischaemic CAP recovery (∼55–60%, Fig. 5B). Taken together, these results indicate that IP3 receptor-dependent Ca2+ release also contributed significantly to dorsal column white matter injury. We reasoned that if all three sources of Ca2+ were restrained (external influx, ryanodine receptor- and IP3 receptor-dependent release of intracellular pools) injury should be largely prevented. This was indeed the case: applying nimodipine (to block depolarization-dependent release of ryanodine-dependent stores; Ouardouz et al.2003) and AIDA (to block signalling that converges onto IP3-dependent Ca2+ stores) in the absence of external Ca2+, completely rescued dorsal columns from 60 min of OGD (CAP area recovery to 97 ± 32% of control, n = 5, versus 4 ± 6% in Ca2+-containing aCSF with no blockers, P < 10−5versus 0 Ca2+; P = 0.027 versus 0Ca2+ + nimodipine, Fig. 5A).
Figure 5. Role of intracellular Ca2+ stores during OGD in spinal cord dorsal column.
A, quantitative summary of mean CAP area recovery in the presence of various agents after 60 min OGD in the absence of bath Ca2+. Blocking Ca2+ release from ryanodine-dependent stores by blocking voltage-gated Ca2+ channels (nimodipine, 10 μm) or ryanodine receptors directly (ryanodine, 50 μm) was highly protective, emphasizing the importance of this Ca2+ source in ischaemic injury. Eliminating Ca2+ influx from the extracellular space while blocking both ryanodine- and IP3-dependent Ca2+ release (0Ca2+ + EGTA + nimodipine + 50 μm AIDA) rendered dorsal columns virtually completely resistant to a severe 1 h ischaemic insult. B, similarly, interfering with Ca2+ release from IP3-dependent Ca2+ stores by blocking group 1 mGluRs (AIDA), inhibiting phospholipase C (and therefore IP3 synthesis; U73122, 20 μm), or blocking IP3 receptors directly (2-APB, 100 μm) was also very protective. These results illustrate the three main Ca2+ sources in ischaemic spinal axons: extracellular space, ryanodine- and IP3-dependent Ca2+ stores. n = 8, 10, 6, 7 and 5 for no drug, nimodipine, ryanodine, nimodipine + ryanodine and nimodipine + AIDA, respectively, as shown in upper bar graph and n = 8, 8, 10 and 5 for no drug, AIDA U73122 and 2APB, respectively, as shown in lower bar graph.
AMPA promotes axoplasmic Ca2+ rise partly from ryanodine-dependent Ca2+ stores
Taken together, the results thus far strongly suggest a mechanistic link between AMPA receptors and intra-axonal Ca2+ stores. To directly demonstrate such a connection, we activated AMPA receptors selectively in uninjured, non-ischaemic dorsal column slices using AMPA (100 μm, plus 30 μm cyclothiazide to reduce receptor desensitization) while imaging axoplasmic Ca2+ changes using Oregon Green-488 BAPTA-1 fluorescence (Fig. 6). After 30 min of exposure, fluorescence increased by 86 ± 53% over baseline (n = 48 axons from seven different experiments). Blocking ryanodine receptors (50 μm ryanodine) significantly reduced (but did not completely abolish) the AMPA-evoked axonal Ca2+ rise (16 ± 34% at 30 min, n = 26 axons from three experiments, P = 10−7). In order to help exclude a potential glial contribution to the effects of AMPA on axonal Ca2+ rise, myelinic Ca2+ was measured in response to application of AMPA + cyclothiazide. After 30 min of exposure, Ca2+-dependent fluorescence did not change significantly (−4 ± 5%, n = 28 myelin regions).
Axoplasmic Ca2+ changes during chemical ischaemia
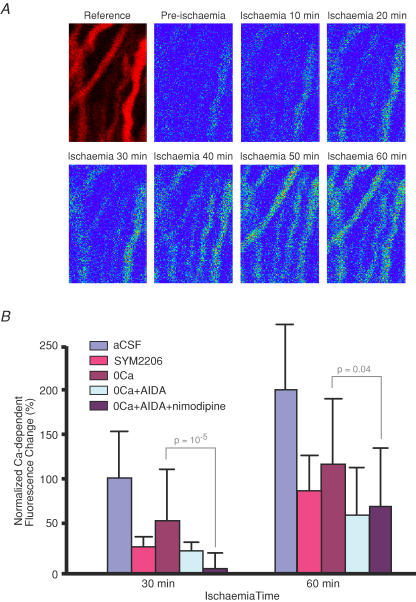
Using confocal microscopy, relative axoplasmic [Ca2+] was then measured during chemical ischaemia at 10-min intervals for 60 min. Representative micrographs are shown in Fig. 7A. In Ca2+-containing perfusate, axoplasmic Ca2+-dependent fluorescence gradually increased to 99 ± 50% above pre-ischaemic baseline after 30 min and 198 ± 68% after 60 min ischaemia (n = 30 axons). In the absence of external Ca2+ (with the addition of 0.5 mm EGTA which caused the extracellular Ca2+ to fall to negligible levels within 4 min; Nikolaeva et al. 2005) ischaemia still induced a rise of axoplasmic [Ca2+], though to a lesser degree (54 ± 54% in 0Ca2+ compared to 99 ± 50% in normal Ca2+ after 30 min, P = 10−4versus Ca2+-containing aCSF, and 116 ± 68% in 0Ca2+ compared to 198 ± 68% in normal Ca2+ after 60 min, P = 7 × 10−5, n = 32 and 30 axons, respectively). These results confirm that significant amounts of Ca2+ are sourced from intracellular compartments during injury in these axons (Ouardouz et al. 2003). Combined application of AIDA and nimodipine in the absence of external Ca2+ almost completely blocked Ca2+ increase after 30 min (5 ± 16% versus 54 ± 54% in 0Ca2+ + EGTA, P = 10−5, n = 45 and 32 axons, respectively). This block was less pronounced but still significant after 60 min of ischaemia (67 ± 60% versus 116 ± 68%, P = 0.04). The AMPA antagonist SYM2206 (30 μm) also significantly reduced ischaemia-induced axonal increase in Ca2+ concentration at 30 min (28 ± 9% versus 99 ± 50% in normal Ca2+-containing aCSF, P = 2.4 × 10−5) and 60 min (86 ± 37% versus 198 ± 68%, P = 3.7 × 10−4).
Figure 7. Confocal imaging of axoplasmic [Ca2+] in spinal cord dorsal column axons during chemical ischaemia.
A, representative micrographs showing axon profiles outlined by a red Ca2+-insensitive dextran-conjugated fluorophore (Reference). Remaining panels show Ca2+-sensitive emission in pseudocolour from pre-ischaemic fibres and at 10-min intervals during ischaemia (induced using NaN3 + iodoacetic acid (IAA)). B, bar graph showing quantitative changes in axoplasmic Ca2+-dependent fluorescence. Ischaemia increased axoplasmic Ca2+ levels in Ca2+-containing perfusate, which was reduced but not prevented by removing bath Ca2+ (with the addition of 0.5 mm EGTA). Addition of AIDA (50 μm) to 0Ca2+ + EGTA perfusate blunted the ischaemic [Ca2+] increase (not significant with respect to 0Ca2+ alone). However, combined application of AIDA and nimodipine (10 μm) in the absence of external Ca2+ significantly blocked axonal [Ca2+] rise. The AMPA antagonist SYM2206 (30 μm) was highly effective even in the presence of bath Ca2+. n = 30, 9, 32, 16 and 45 for aCSF, SYM2206, 0Ca, 0Ca2+ + AIDA, 0Ca2+ + AIDA + nimodipine.
Immunolocalization of AMPA receptors
Dorsal columns were immunostained with antibodies specific for GluR1, GluR2/3 and GluR4, the main subunits making up AMPA receptors (Fig. 8). Axon cylinders were labelled by neurofilament antibody which is shown as red in Fig. 8. GluR1 staining was patchy and mostly appeared just outside larger axons, in regions of the myelin sheath. GluR2/3 (probably GluR3 only, see legend to Fig. 8) and GluR4 signals on the other hand were punctate, and were localized at the edges of neurofilament-positive axonal profiles, suggesting expression on the inner myelin loops and/or the axolemma.
Figure 8. Rat dorsal column sections were immunostained with antibodies recognizing the GluR1–4 AMPA receptor subunits (green).
Axon cylinders are identified by neurofilament (red). GluR1 staining was patchy but was seen throughout the thickness of the myelin sheath. In contrast, GluR2/3 and GluR4 staining was punctate, and was localized at the edges of neurofilament-positive axonal profiles. Given the absence of GluR2 subunits in rat spinal white matter shown previously (Park et al. 2003), our GluR2/3 label most probably reflects the presence of GluR3 subunits. The limited resolution of light microscopy does not permit us to conclusively localize the GluR3 and 4 clusters to inner myelin loops versus axolemma. Scale bars, 5 μm.
Discussion
Acute energy failure induced by OGD caused irreversible damage to spinal cord dorsal columns. In contrast to the situation with anoxia (Imaizumi et al. 1997) or trauma (Agrawal & Fehlings, 1996), here we show that inhibition of voltage-gated Na+ channels by TTX or removal of external Na+ was not protective against 60 min of OGD. This discrepancy is likely to be due to the difference in severity of the insults. It is interesting that 0Na+ solution rescued spinal cord dorsal columns from a shorter (30 min) OGD exposure whereas TTX was ineffective (Fig. 1). This indicates that shorter ischaemic injury is heavily dependent on extracellular Na+ ions, but that Na+ influx pathways other than TTX-sensitive Na+ channels play an important role. These results underscore the relatively minor role of voltage-gated Na+ channels in dorsal column ischaemia compared to other CNS white matter tracts such as optic nerve (see Fig. 7 in Stys, 2004), even though dorsal column axons appear to exhibit a resting non-inactivating permeability to Na+ that is similar in magnitude to that in optic nerve (Fig. 2B; Leppanen & Stys, 1997a). Similarly, removal of extracellular Ca2+ protected against 30 min, but was ineffective after 60 min of OGD; removal of both Na+ and Ca2+ ions from the bath was necessary to protect dorsal columns from longer OGD exposures (Ouardouz et al. 2003). Analysis of resting membrane potentials during ischaemia indicates that voltage-gated Na+ channels are not the sole mediators of depolarization: in contrast to the resting state, where AMPA receptors appear not to contribute to resting membrane potential, during ischaemia these receptors become activated and contribute to ischaemic depolarization as much if not more than Na+ channels (Fig. 2C). Indeed, adding AMPA receptor blockade to TTX significantly reduced the degree of ischaemic axonal depolarization at 30 and 60 min (Fig. 2C), indicating that AMPA receptors, in addition to compromising glia, may represent an important route for Na+ influx into ischaemic dorsal column axons. Moreover, the degree of depolarization at 30 and 60 min of ischaemia in the presence of TTX + SYM2206 was identical to that observed in 0Na+ perfusate, suggesting that together, Na+ channels and AMPA receptors may represent the two major avenues of Na+ flux that in turn promote axonal depolarization in ischaemic dorsal columns.
The data above indicate that as the injury intensifies, an increasingly complex interplay of damaging Na+- and Ca2+-dependent pathways occurs. In light of this, it is remarkable that AMPA receptor inhibitors were so protective against prolonged (60 min) OGD even in Ca2+-containing perfusate, a protocol where total Ca2+ or Na+ removal was completely ineffective. Although GYKI52466 and SYM2206 have been extensively used as selective AMPA receptor antagonists (Ouardouz & Durand, 1991; Donevan & Rogawski, 1993; Pei et al. 1999), we found that both agents also blocked currents carried by Nav1.6 at concentrations routinely used to inhibit AMPA receptors (C. E. Morris, W. Lin and P. K. Stys, unpublished observations). The protective effect obtained by GYKI52466 and SYM2206 was similar to kynurenic acid and CNQX. Therefore, the actions of GYKI52466 and SYM2206 in this study are ascribable mainly to AMPA receptor-dependent mechanisms. In addition, TTX and removal of external Na+ were completely ineffective; therefore, it is unlikely that the neuroprotective effects of GYKI52466 and SYM2206 were due to Nav1.6 block. Taken together, our data are consistent with the notion that during prolonged dorsal column ischaemia, transaxolemmal Na+ and Ca2+ flux, and secondary release of Ca2+ from Ca2+ stores (Ouardouz et al. 2003), appear to be governed by AMPA receptors to a significant degree. Moreover, the glutamate transport blocker dihydrokainate had similar effects, demonstrating that this pathway can be blocked either at the receptor end, or at the point of transmitter release (Li et al. 1999). Even then, there remained ∼40% electrophysiological injury that is under the control of distinct signalling pathways (see below).
AMPA receptor activation has been reported to be involved in white matter injury (Agrawal & Fehlings, 1997; Wrathall et al. 1997; Li et al. 1999; Rosenberg et al. 1999; Tekkok & Goldberg, 2001). Whereas the precise mechanisms have not been fully elucidated, the presence of these receptors on both astrocytes and oligodendrocytes (Li & Stys, 2000; Verkhratsky & Steinhauser, 2000), and their ability to damage these cells (Oka et al. 1993; David et al. 1996; Matute et al. 1997; McDonald et al. 1998; Chen et al. 2000; Li & Stys, 2000), suggests that at least part of the injury observed in traumatic, anoxic and ischaemic spinal white matter may be due to disruption of glial elements. Studies in white matter from different central nervous system regions have shown AMPA/kainate receptors in oligodendrocytes, astrocytes and axons (Matute & Miledi, 1993; Patneau et al. 1994; Garcia-Barcina & Matute, 1996; Agrawal & Fehlings, 1997; Li & Stys, 2000; Brand-Schieber & Werner, 2003). Accordingly, one explanation for the axonal protective effect of AMPA receptor antagonists was suggested to be secondary to sparing of glial elements, leading to a reduction of diffusible messengers (e.g. NO and free radicals) (for review see Matute et al. 2001). However, exposure of dorsal columns to kainate (an AMPA and kainate receptor agonist) induces axonal rises in [Ca2+]; addition of extracellular scavengers of NO and other free radicals does not abolish the kainate-induced [Ca2+] increase (Ouardouz & Stys, 2004). Moreover, the highly localized expression of clusters of GluR3 and GluR4 immediately adjacent to neurofilament profiles (Fig. 8), together with the observed membrane potential measurements (which reflect purely axonal, and not glial potentials), the AMPA-inducible release of Ca2+ from axonal ryanodine-dependent stores, and the lack of myelinic Ca2+ increase in response to AMPA receptor activation, suggest that in addition to a potential action on glial cells that cannot be excluded, AMPA receptors may directly mediate injury to axons.
The present data confirm our previous findings of ryanodine receptor-dependent ischaemic injury (Ouardouz et al. 2003) and further extend this finding by implicating IP3 receptor-dependent Ca2+ stores; interfering with this pathway at three separate points (group 1 mGluR block with AIDA, inhibition of phospholipases C with U73122 or IP3 receptor block with 2APB) was highly protective in 0Ca2+ perfusate (Fig. 5B). Indeed, removing external Ca2+ and restraining both ryanodine- and IP3-dependent stores in combination (0Ca2+ + nimodipine + AIDA, Fig. 5A) was completely protective against a very severe 1 h ischaemic insult; this identifies the three most important sources of noxious Ca2+ mobilized by this protocol. The second surprising finding was the potent protective effect of AMPA receptor antagonists vis-à-vis the ineffectiveness of bath Ca2+ removal. This observation, together with the injurious effects of intracellular Ca2+ release, and ryanodine-blockable axonal Ca2+ increase induced by AMPA (Fig. 6), point to an intimate link between AMPA receptor signalling and axonal Ca2+ stores. In addition to admitting Ca2+ from the extracellular space, AMPA receptors may control internal Ca2+ pools by two potential mechanisms. The first may involve flux of Na+ into axons which stimulates release of Ca2+ from ryanodine- and IP3-dependent Ca2+ stores in optic nerve (Nikolaeva et al. 2005). Indeed, given that TTX-sensitive Na+ channels contribute to resting axonal membrane potential, whereas AMPA receptors do not (Fig. 2C), it is conceivable that the former initiate axonal Na+ loading and depolarization early in ischaemia, triggering initial glutamate release leading to activation of AMPA receptors. These then assume a dominant role in Na+ influx, causing axonal depolarization and more glutamate release, thus establishing a positive feedback loop. The other intriguing possibility is that the traditionally ionotropic AMPA receptors signal in a ‘non-canonical’ manner, by exerting a modulatory influence rather than fluxing ions. Metabotropic actions have been recently reported for both AMPA (Satake et al. 2004; Takago et al. 2005) and kainate receptors (Melyan et al. 2002; Rozas et al. 2003) mediated in part via a G-protein, phospholipase C-dependent pathway (Rodriguez-Moreno & Lerma, 1998). Our results using the phospholipase C inhibitor U73122 may in fact reflect such a mechanism, stimulated by metabotropic behaviour of AMPA receptors. Additional studies are required to definitively prove a non-ionotropic action of AMPA receptors in spinal white matter. It is also highly likely that such a runaway release of axoplasmic glutamate (Li et al. 1999) also activates NMDA receptors on adjacent adaxonal myelin (Micu et al. 2006) and neighbouring oligodendrocytes (Karadottir et al. 2005; Salter & Fern, 2005), promoting damage to these elements.
Confocal microscopy revealed a sustained increase of intra-axonal [Ca2+] during 1 h of in vitro ischaemia; removal of extracellular Ca2+ reduced but did not eliminate the intra-axonal Ca2+ increase, which was previously shown to be sufficient to irreversibly damage this tissue (Ouardouz et al. 2003, 2005). One of the mechanisms by which Ca2+ is released from stores in dorsal column axons involves activation of ryanodine receptors by axonal L-type Ca2+ channels gated by ischaemic depolarization (Ouardouz et al. 2003). A second major intracellular Ca2+ pool is controlled by IP3 receptors. Activation of phospholipase C by certain G-protein-coupled metabotropic receptors (Pin & Duvoisin, 1995; Recasens & Vignes, 1995), including type 1 metabotropic glutamate receptors, induces formation of IP3 and diacylglycerol (Bockaert et al. 1993). Our electrophysiological and Ca2+ imaging experiments are consistent with the idea of multiple Ca2+ sources as described above. Our results also emphasize that additional deleterious pathways are recruited as the insult grows more severe, and restraining any one or even several is necessary but may not be sufficient to confer overall functional protection. Indeed, even blocking multiple pathways of Ca2+ accumulation still allowed a substantial late rise in [Ca2+] (Fig. 7), suggesting yet additional undefined axonal Ca2+ sources recruited by longer insults. Finally, our data strongly support a central role of AMPA receptor activation during dorsal column ischaemia, which leads to functional injury and a deleterious axoplasmic rise in [Ca2+]; inhibition of these receptors confers potent neuroprotection in central white matter.
Acknowledgments
This work was supported by National Institute of Neurological Disorders and Stroke (NINDS) R01 NS040087-06; operating grant no. MOP-37858 and equipment grant no. MMA-48299 from the Canadian Institutes of Health Research. P.K.S. was supported by a Heart and Stroke Foundation of Ontario Career Investigator Award.
References
- Agrawal SK, Fehlings MG. Mechanisms of secondary injury to spinal cord axons in vitro: role of Na+, Na+-K+-ATPase, the Na+-H+ exchanger, and the Na+-Ca2+ exchanger. J Neurosci. 1996;16:545–552. doi: 10.1523/JNEUROSCI.16-02-00545.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal SK, Fehlings MG. Role of NMDA and non-NMDA ionotropic glutamate receptors in traumatic spinal cord axonal injury. J Neurosci. 1997;17:1055–1063. doi: 10.1523/JNEUROSCI.17-03-01055.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal SK, Theriault E, Fehlings MG. Role of group I metabotropic glutamate receptors in traumatic spinal cord white matter injury. J Neurotrauma. 1998;15:929–941. doi: 10.1089/neu.1998.15.929. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Pin J, Fagni L. Metabotropic glutamate receptors: an original family of G protein-coupled receptors. Fundam Clin Pharmacol. 1993;7:473–485. doi: 10.1111/j.1472-8206.1993.tb00252.x. [DOI] [PubMed] [Google Scholar]
- Brand-Schieber E, Werner P. (±)-α-Amino-3-hydroxy-5-methylisoxazole-4-propionic acid and kainate receptor subunit expression in mouse versus rat spinal cord white matter: similarities in astrocytes but differences in oligodendrocytes. Neurosci Lett. 2003;345:126–130. doi: 10.1016/s0304-3940(03)00469-5. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Liao SL, Kuo JS. Gliotoxic action of glutamate on cultured astrocytes. J Neurochem. 2000;75:1557–1565. doi: 10.1046/j.1471-4159.2000.0751557.x. [DOI] [PubMed] [Google Scholar]
- David JC, Yamada KA, Bagwe MR, Goldberg MP. AMPA receptor activation is rapidly toxic to cortical astrocytes when desensitization is blocked. J Neurosci. 1996;16:200–209. doi: 10.1523/JNEUROSCI.16-01-00200.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donevan SD, Rogawski MA. GYKI 52466, a 2,3-benzodiazepine, is a highly selective, noncompetitive antagonist of AMPA/kainate receptor responses. Neuron. 1993;10:51–59. doi: 10.1016/0896-6273(93)90241-i. [DOI] [PubMed] [Google Scholar]
- Fern R, Ransom BR, Stys PK, Waxman SG. Pharmacological protection of CNS white matter during anoxia: actions of phenytoin, carbamazepine and diazepam. J Pharmacol Exp Ther. 1993;266:1549–1555. [PubMed] [Google Scholar]
- Fern R, Ransom BR, Waxman SG. Voltage-gated calcium channels in CNS white matter: role in anoxic injury. J Neurophysiol. 1995;74:369–377. doi: 10.1152/jn.1995.74.1.369. [DOI] [PubMed] [Google Scholar]
- Garcia-Barcina JM, Matute C. Expression of kainate-selective glutamate receptor subunits in glial cells of the adult bovine white matter. Eur J Neurosci. 1996;8:2379–2387. doi: 10.1111/j.1460-9568.1996.tb01201.x. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Hartley M, Heinemann S. Ca2+ permeability of KA-AMPA-gated glutamate receptor channels depends on subunit composition. Science. 1991;252:851–853. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- Imaizumi T, Kocsis JD, Waxman SG. Anoxic injury in the rat spinal cord: pharmacological evidence for multiple steps in Ca2+-dependent injury of the dorsal columns. J Neurotrauma. 1997;14:299–311. doi: 10.1089/neu.1997.14.299. [DOI] [PubMed] [Google Scholar]
- Imaizumi T, Kocsis JD, Waxman SG. The role of voltage-gated Ca2+ channels in anoxic injury of spinal cord white matter. Brain Res. 1999;817:84–92. doi: 10.1016/s0006-8993(98)01214-1. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Stys PK. Calpain inhibitors confer biochemical, but not electrophysiological, protection against anoxia in rat optic nerves. J Neurochem. 2000;74:2101–2107. doi: 10.1046/j.1471-4159.2000.0742101.x. [DOI] [PubMed] [Google Scholar]
- Karadottir R, Cavelier P, Bergersen LH, Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438:1162–1166. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauppinen RA, Nicholls DG. Failure to maintain glycolysis in anoxic nerve terminals. J Neurochem. 1986;47:1864–1869. doi: 10.1111/j.1471-4159.1986.tb13100.x. [DOI] [PubMed] [Google Scholar]
- Leppanen LL, Stys PK. Ion transport and membrane potential in CNS myelinated axons. I: normoxic conditions. J Neurophysiol. 1997a;78:2086–2094. doi: 10.1152/jn.1997.78.4.2086. [DOI] [PubMed] [Google Scholar]
- Leppanen L, Stys PK. Ion transport and membrane potential in CNS myelinated axons. II. Effects of metabolic inhibition. J Neurophysiol. 1997b;78:2095–2107. doi: 10.1152/jn.1997.78.4.2095. [DOI] [PubMed] [Google Scholar]
- Li S, Mealing GA, Morley P, Stys PK. Novel injury mechanism in anoxia and trauma of spinal cord white matter: glutamate release via reverse Na+-dependent glutamate transport. J Neurosci. 1999;19:RC16. doi: 10.1523/JNEUROSCI.19-14-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Stys PK. Mechanisms of ionotropic glutamate receptor-mediated excitotoxicity in isolated spinal cord white matter. J Neurosci. 2000;20:1190–1198. doi: 10.1523/JNEUROSCI.20-03-01190.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JW, Althomsons SP, Hyrc KL, Choi DW, Goldberg MP. Oligodendrocytes from forebrain are highly vulnerable to AMPA/kainate receptor-mediated excitotoxicity. Nat Med. 1998;4:291–297. doi: 10.1038/nm0398-291. [DOI] [PubMed] [Google Scholar]
- Malek SA, Coderre E, Stys PK. Aberrant chloride transport contributes to anoxic/ischemic white matter injury. J Neurosci. 2003;23:3826–3836. doi: 10.1523/JNEUROSCI.23-09-03826.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute C, Alberdi E, Domercq M, Perez-Cerda F, Perez-Samartin A, Sanchez-Gomez MV. The link between excitotoxic oligodendroglial death and demyelinating diseases. Trends Neurosci. 2001;24:224–230. doi: 10.1016/s0166-2236(00)01746-x. [DOI] [PubMed] [Google Scholar]
- Matute C, Miledi R. Neurotransmitter receptors and voltage-dependent Ca2+ channels encoded by mRNA from the adult corpus callosum. Proc Natl Acad Sci U S A. 1993;90:3270–3274. doi: 10.1073/pnas.90.8.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute C, Sanchez-Gomez M, Martinez-Millán L, Miledi R. Glutamate receptor-mediated toxicity in optic nerve oligodendrocytes. Proc Natl Acad Sci U S A. 1997;94:8830–8835. doi: 10.1073/pnas.94.16.8830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melyan Z, Wheal HV, Lancaster B. Metabotropic-mediated kainate receptor regulation of IsAHP and excitability in pyramidal cells. Neuron. 2002;34:107–114. doi: 10.1016/s0896-6273(02)00624-4. [DOI] [PubMed] [Google Scholar]
- Micu I, Jiang Q, Coderre E, Ridsdale A, Zhang L, Woulfe J, Yin X, Trapp BD, McRory JE, Rehak R, Zamponi GW, Wang W, Stys PK. NMDA receptors mediate calcium accumulation in myelin during chemical ischemia. Nature. 2006;439:988–992. doi: 10.1038/nature04474. [DOI] [PubMed] [Google Scholar]
- Nikolaeva MA, Mukherjee B, Stys PK. Na+-dependent sources of intra-axonal Ca2+ release in rat optic nerve during in vitro chemical ischemia. J Neurosci. 2005;25:9960–9967. doi: 10.1523/JNEUROSCI.2003-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka A, Belliveau MJ, Rosenberg PA, Volpe JJ. Vulnerability of oligodendroglia to glutamate: pharmacology, mechanisms, and prevention. J Neurosci. 1993;13:1441–1453. doi: 10.1523/JNEUROSCI.13-04-01441.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouardouz M, Durand J. GYKI 52466 antagonizes glutamate responses but not NMDA and kainate responses in rat abducens motoneurones. Neurosci Lett. 1991;125:5–8. doi: 10.1016/0304-3940(91)90115-a. [DOI] [PubMed] [Google Scholar]
- Ouardouz M, Nikolaeva MA, Coderre E, Zamponi GW, McRory JE, Trapp BD, Yin X, Wang W, Woulfe J, Stys PK. Depolarization-induced Ca2+ release in ischemic spinal cord white matter involves L-type Ca2+ channel activation of ryanodine receptors. Neuron. 2003;40:53–63. doi: 10.1016/j.neuron.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Ouardouz M, Stys PK. Program No. 99.8 2004 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience 2004; 2004. Axonal AMPA receptors promote Ca increase in rat spinal cord axons. Online. [Google Scholar]
- Ouardouz M, Zamponi GW, Barr W, Kiedrowski L, Stys PK. Protection of ischemic rat spinal cord white matter: Dual action of KB-R7943 on Na+/Ca2+ exchange and L-type Ca2+ channels. Neuropharmacology. 2005;48:566–575. doi: 10.1016/j.neuropharm.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Park E, Liu Y, Fehlings MG. Changes in glial cell white matter AMPA receptor expression after spinal cord injury and relationship to apoptotic cell death. Exp Neurol. 2003;182:35–48. doi: 10.1016/s0014-4886(03)00084-0. [DOI] [PubMed] [Google Scholar]
- Patneau DK, Wright PW, Winters C, Mayer ML, Gallo V. Glial cells of the oligodendrocyte lineage express both kainate- and AMPA-preferring subtypes of glutamate receptor. Neuron. 1994;12:357–371. doi: 10.1016/0896-6273(94)90277-1. [DOI] [PubMed] [Google Scholar]
- Pei XF, Sturgess MA, Valenzuela CF, Maccecchini ML. Allosteric modulators of the AMPA receptor: novel 6-substituted dihydrophthalazines. Bioorg Med Chem Lett. 1999;9:539–542. doi: 10.1016/s0960-894x(99)00044-x. [DOI] [PubMed] [Google Scholar]
- Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- Recasens M, Vignes M. Excitatory amino acid metabotropic receptor subtypes and calcium regulation. Ann N Y Acad Sci. 1995;757:418–429. doi: 10.1111/j.1749-6632.1995.tb17501.x. [DOI] [PubMed] [Google Scholar]
- Ren Y, Risdale A, Corderre E, Stys PK. Calcium imaging in live rat optic nerve myelinated axons in vitro using confocal laser microscopy. J Neurosci Methods. 2000;102:165–176. doi: 10.1016/s0165-0270(00)00304-6. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Moreno A, Lerma J. Kainate receptor modulation of GABA release involves a metabotropic function. Neuron. 1998;20:1211–1218. doi: 10.1016/s0896-6273(00)80501-2. [DOI] [PubMed] [Google Scholar]
- Rosenberg LJ, Teng YD, Wrathall JR. 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo (f) quinoxaline reduces glial loss and acute white matter pathology after experimental spinal cord contusion. J Neurosci. 1999;19:464–475. doi: 10.1523/JNEUROSCI.19-01-00464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozas JL, Paternain AV, Lerma J. Noncanonical signaling by ionotropic kainate receptors. Neuron. 2003;39:543–553. doi: 10.1016/s0896-6273(03)00436-7. [DOI] [PubMed] [Google Scholar]
- Sabri MI, Ochs S. Inhibition of glyceraldehyde-3-phosphate dehydrogenase in mammalian nerve iodoacetic acid. J Neurochem. 1971;18:1509–1514. doi: 10.1111/j.1471-4159.1971.tb00013.x. [DOI] [PubMed] [Google Scholar]
- Salter MG, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature. 2005;438:1167–1171. doi: 10.1038/nature04301. [DOI] [PubMed] [Google Scholar]
- Satake S, Saitow F, Rusakov D, Konishi S. AMPA receptor-mediated presynaptic inhibition at cerebellar GABAergic synapses: a characterization of molecular mechanisms. Eur J Neurosci. 2004;19:2464–2474. doi: 10.1111/j.0953-816X.2004.03347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stämpfli R. A new method for measuring membrane potentials with external electrodes. Experientia. 1954;10:508–509. doi: 10.1007/BF02166189. [DOI] [PubMed] [Google Scholar]
- Stys PK. White matter injury mechanisms. Curr Mol Med. 2004;4:113–130. doi: 10.2174/1566524043479220. [DOI] [PubMed] [Google Scholar]
- Stys PK, Ransom BR, Waxman SG. Compound action potential of nerve recorded by suction electrode: a theoretical and experimental analysis. Brain Res. 1991;546:18–32. doi: 10.1016/0006-8993(91)91154-s. [DOI] [PubMed] [Google Scholar]
- Stys PK, Sontheimer H, Ransom BR, Waxman SG. Noninactivating, tetrodotoxin-sensitive Na+ conductance in rat optic nerve axons. Proc Natl Acad Sci U S A. 1993;90:6976–6980. doi: 10.1073/pnas.90.15.6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stys PK, Waxman SG, Ransom BR. Ionic mechanisms of anoxic injury in mammalian CNS white matter: role of Na+-channels and Na+-Ca2+ exchanger. J Neurosci. 1992;12:430–439. doi: 10.1523/JNEUROSCI.12-02-00430.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takago H, Nakamura Y, Takahashi T. G protein-dependent presynaptic inhibition mediated by AMPA receptors at the calyx of Held. Proc Natl Acad Sci U S A. 2005;102:7368–7373. doi: 10.1073/pnas.0408514102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekkok SB, Goldberg MP. Ampa/kainate receptor activation mediates hypoxic oligodendrocyte death and axonal injury in cerebral white matter. J Neurosci. 2001;21:4237–4248. doi: 10.1523/JNEUROSCI.21-12-04237.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng YD, Wrathall JR. Local blockade of sodium channels by tetrodotoxin ameliorates tissue loss and long-term functional deficits resulting from experimental spinal cord injury. J Neurosci. 1997;17:4359–4366. doi: 10.1523/JNEUROSCI.17-11-04359.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbny Y, Zhang C-L, Chiu SY. Coupling of calcium homeostasis to axonal sodium in axons of mouse optic nerve. J Neurophysiol. 2002;88:802–816. doi: 10.1152/jn.2002.88.2.802. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Steinhauser C. Ion channels in glial cells. Res Brain Res Rev. 2000;32:380–412. doi: 10.1016/s0165-0173(99)00093-4. [DOI] [PubMed] [Google Scholar]
- Wolf JA, Stys PK, Lusardi T, Meaney D, Smith DH. Traumatic axonal injury induces calcium influx modulated by tetrodotoxin-sensitive sodium channels. J Neurosci. 2001;21:1923–1930. doi: 10.1523/JNEUROSCI.21-06-01923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrathall JR, Teng YD, Marriott R. Delayed antagonism of AMPA/kainate receptors reduces long-term functional deficits resulting from spinal cord trauma. Exp Neurol. 1997;145:565–573. doi: 10.1006/exnr.1997.6506. [DOI] [PubMed] [Google Scholar]