Abstract
The aim of this study was to determine how ATP synthesis and contractility in vivo are altered by ischaemia in working human skeletal muscle. The hypotheses were: (1) glycolytic flux would be higher during ischaemic (ISC) compared to free-flow (FF) muscle contractions, in compensation for reduced oxidative ATP synthesis, and (2) ischaemic muscle fatigue would be related to the accumulation of inhibitory metabolic by-products rather than to the phosphorylation potential ([ATP]/[ADP][Pi]) of the muscle. Twelve healthy adults (6 men, 6 women) performed six intermittent maximal isometric contractions of the ankle dorsiflexors (12 s contract, 12 s relax), once with intact blood flow and once with local ischaemia by thigh cuff inflation to 220 Torr. Intracellular phosphorous metabolites and pH were measured non-invasively with magnetic resonance spectroscopy, and rates of ATP synthesis through oxidative phosphorylation, anaerobic glycolysis, and the creatine kinase reaction were determined. The force–time integral declined more during ISC (66 ± 3% initial) than FF (75 ± 2% initial, P = 0.002), indicating greater fatigue in ISC. [ATP] was preserved in both protocols, indicating matching of ATP production and use under both conditions. Glycolytic flux (mm s−1) was similar during FF and ISC (P = 0.16). Total ATP synthesis rate was lower during ISC, despite adjustment for the greater muscle fatigue in this condition (P < 0.001). Fatigue was linearly associated with diprotonated inorganic phosphate (FF r = 0.94 ± 0.01, ISC r = 0.92 ± 0.02), but not phosphorylation potential. These data provide novel evidence that ATP supply and demand in vivo are balanced in human skeletal muscle during ischaemic work, not through higher glycolytic flux, but rather through increased metabolic economy and decreased rates of ATP consumption as fatigue ensues.
Chemical energy in the form of adenosine triphosphate (ATP) is essential to maintain function in contracting skeletal muscle cells. Despite the approximately 100-fold increase in ATP consumption from rest to maximal-intensity contractions, the energetic demands of muscle are usually satisfied without depleting intracellular ATP (Dawson et al. 1977). Under most conditions, the demand for ATP is matched by ATP production from mitochondrial oxidative phosphorylation and anaerobic glycolysis, with temporal buffering from the creatine kinase reaction.
During steady-state muscle activity, where the supply of oxygen and other substrates to the mitochondria are not limited, the rate of oxidative phosphorylation increases in proportion to increases in muscle work (i.e. ATPase activity) (Chance et al. 1985). During ischaemia, however, the contribution of oxidative phosphorylation to the ATP pool will diminish as intracellular oxygen tension (PO2) falls below the level required to support mitochondrial ATP synthesis (Connett et al. 1990). It has been suggested that, under these conditions, the balance between ATP supply and demand can be accomplished by increasing reliance on ATP synthesis via substrate-level phosphorylation, which assumes that the glycolytic rate is well below the capacity for this pathway in vivo. While there is some evidence that muscle ATP supply is maintained during ischaemia by a compensatory increase in glycolytic flux (King et al. 1987; Kemp et al. 1994; Timmons et al. 1996), this finding is not universal (Conley et al. 1998; Hogan et al. 1998) and appears to depend on muscle fibre type (Greenhaff et al. 1993), contraction intensity (Linnarsson et al. 1974), and the precise manner in which oxygen delivery to the working muscle is limited (King et al. 1987; Gutierrez et al. 1988; Stainsby et al. 1990).
In contrast to this concept of an energetic adjustment to ischaemia, several investigators have suggested that the balance between ATP supply and demand in ischaemic muscle is accomplished by reducing force development, and thus decreasing the demand by the ATPases (Hultman & Sjoholm, 1983; Van der Laarse et al. 1989; Hogan et al. 1996; Meyer & Foley, 1996). Indeed, many investigators have reported greater muscle fatigue, defined as an exercise-induced reduction in muscle force-generating capacity, during ischaemic compared to free-flow contractions (Harris et al. 1975; Greenhaff et al. 1993; Kemp et al. 1994; Hogan et al. 1999). However, greater fatigue during ischaemia may result from direct inhibition of contractile processes by the metabolic products consequent to increased glycolytic flux (Fabiato & Fabiato, 1978). Alternatively, a lower cytosolic phosphorylation potential ([ATP]/[ADP][Pi]), a proxy for the free energy of ATP hydrolysis (ΔGATP), may act as a thermodynamic ‘braking function’ on ATP turnover, thereby causing muscle force production to decline as ATP hydrolysis becomes less favourable (Arthur et al. 1992; Meyer & Foley, 1996). The former possibility is supported by studies in vitro demonstrating the direct effects of diprotonated Pi on muscle contractility (Nosek et al. 1987), and correlations in vivo between fatigue and H2PO4− (Miller et al. 1988), which accumulates in the muscle during glycolytic ATP turnover. The latter possibility is supported by reports of tight coupling between ATP supply and muscle force production (Hultman & Sjoholm, 1983; Dawson et al. 1978), particularly in the absence of inhibitory metabolite accumulation (Hogan et al. 1996).
The aim of the present study was to determine if a balance between ATP supply and demand is achieved during ischaemic muscle contractions in vivo, and to investigate the following potential mechanisms for this balance: (1) increased glycolytic flux in comparison to free-flow conditions, (2) decreased ATP demand (i.e. fatigue) induced by the accumulation of inhibitory metabolites, or (3) fatigue induced by the coupling of ATP demand to ATP supply, mediated by decreased ATP turnover in response to a reduced intracellular phosphorylation potential and independent of inhibitory metabolite levels. We hypothesized that (1) glycolytic flux would be higher during ischaemic compared to free-flow muscle contractions, presumably in compensation for reduced oxidative ATP synthesis, and (2) muscle fatigue would be related to accumulation of inhibitory metabolites rather than the intracellular phosphorylation potential.
Methods
Subjects
Twelve healthy, untrained subjects were enrolled in this study (6 men, 6 women, age 21–35 years). Written informed consent was obtained from all subjects, as approved by the University of Massachusetts, Amherst and the Yale University School of Medicine human subjects review boards. The study conformed with the Declaration of Helsinki.
Experimental design
Subjects performed two ankle dorsiflexion exercise protocols consisting of six 12 s isometric maximal voluntary contractions (MVC) with 12 s rest intervals between each MVC. The protocol was performed on one leg with intact blood flow (free-flow, FF) and again on the contralateral leg with circulatory occlusion (ischaemia, ISC). Approximately 1 h of rest was allowed between protocols, and the protocol was randomized as to which leg was tested first. Intracellular pH and phosphorous metabolites were measured continuously during rest, contractions, and a 10 min recovery period using phosphorous magnetic resonance spectroscopy (31P-MRS). These data were used to determine the rates of ATP synthesis via oxidative phosphorylation (ATPOX), anaerobic glycolysis (ATPGLY), and PCr breakdown (ATPCK).
Subjects were tested on two occasions, separated by at least 48 h. The first testing session took place in the Muscle Physiology Laboratory at the University of Massachusetts, where subjects were familiarized with the contraction protocols. We also assessed the degree of voluntary activation during baseline MVCs and at the end of the FF and ISC protocols. The second session took place in the Magnetic Resonance Research Center at Yale University, where subjects repeated both exercise protocols with simultaneous measures of force and intramuscular energy metabolism.
Preliminary testing
Subjects were positioned supine with their leg secured to a custom-built device that allowed measurements of ankle dorsiflexion force, surface electromyography (EMG), and electrical stimulation of the peroneal nerve as previously described (Russ & Kent-Braun, 2003). The foot was secured to a force transducer with a Velcro strap across the metatarso-phalangeal joint. A thermoplastic foot mould was used to provide an even dispersion of force across the foot. The lower leg was further immobilized with a strap just distal to the knee to minimize movement during exercise. A pair of 10 mm diameter stimulating electrodes was affixed over the common peroneal nerve, just distal to the fibular head. A recording EMG electrode was placed over the belly of the tibialis anterior muscle with the reference electrode over the distal tendon. Analog signals corresponding to force and EMG were acquired and digitized at 500 Hz using customized Labview software (National Instruments, Austin, TX, USA). Prior to the ISC trials, a blood pressure cuff was placed around the proximal thigh and connected to a rapid cuff inflator (Hokanson, Inc., Belleview, WA, USA).
Subjects performed three brief MVCs (3–4 s duration) to assess strength, with 2 min of rest between trials. Peak force from the three trials was taken as baseline MVC. The ability to achieve full voluntary activation was assessed by measuring the central activation ratio (Kent-Braun & Le Blanc, 1996). Briefly, a train of supramaximal electrical stimuli (50 Hz, 500 ms) was imposed during the third baseline MVC. A central activation ratio was calculated as the ratio of peak voluntary force to the peak force produced during the superimposed tetanus. A ratio less than 1.0 indicates incomplete voluntary activation.
Following baseline MVC and central activation measures, subjects performed either the FF or ISC contraction protocol. Strong verbal encouragement was provided consistently to all subjects. Subjects also received visual feedback of their force output from a series of light-emitting diodes that were scaled to the baseline MVC. During the last 2 s of the final contraction, a stimulus train was imposed to assess central activation. The protocol was repeated on the contralateral leg with a thigh cuff inflated to 220 Torr. The cuff was inflated for 30 s prior to the start of the contraction series. Muscle fatigue during FF and ISC protocols was defined as the relative decline in the force–time integral (FTI) of each 12 s contraction: fatigue = FTI/FTIinitial × 100.
Metabolic testing
Subjects fasted for 4 h prior to the test and then consumed a nutritional supplement bar (22 g carbohydrate, 6 g fat, 15 g protein) approximately 30 min prior to the test. An MR probe assembly consisting of a 6 cm diameter 1H surface coil and a coplanar 3 cm × 5 cm elliptical 31P surface coil was secured over the tibialis anterior muscle. Subjects were positioned supine in a 4.0 tesla, 72 cm clear bore superconducting magnet (Bruker Biospin, Rheinstetten, Germany) interfaced with Paravision software. The foot was secured in a non-magnetic exercise apparatus, as described for the preliminary testing. Transverse gradient echo scout images of the lower leg were acquired to ensure correct positioning of the subject in the isocentre and to select a region of interest within the tibialis anterior muscle for localized shimming on the muscle water peak using the FASTMAP method. This shimming procedure yielded a full width at half-maximal height of the unfiltered PCr peak of 13 ± 8 Hz (mean ± s.d., all subjects).
While positioned in the magnet, but prior to acquisition of 31P-MRS data, subjects performed two to three brief (3–4 s) MVCs separated by 2 min of rest to establish MVC force for that leg and to provide a standardized warm-up to allow for the potentiation of blood flow in response to muscle contractions (Wigmore et al. 2004). Two minutes after these baseline MVCs were completed, subjects performed the series of six 12 s MVCs with 12 s rest intervals under FF conditions, followed by a 10 min recovery period. Verbal encouragement and visual feedback were provided to subjects as described for preliminary testing. The subject was then removed from the magnet and repositioned to study the opposite leg after a short rest period (20–60 min). Proton imaging, shimming, and baseline MVCs were repeated. The series of 12 s contractions was then performed with circulatory occlusion, as described for preliminary testing.
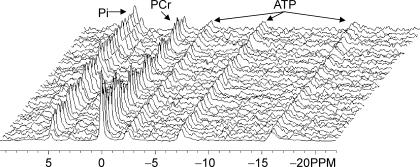
31P-MR spectra (125 μs hard pulse, nominal 60 deg flip angle, 2 s repetition time, 2048 data points, 8000 Hz spectral width) were obtained continuously during 1 min of rest before the contraction protocols and during the FF and ISC protocols and subsequent 10 min recovery periods. The free induction decays were then averaged to yield temporal resolution of 1 min at rest, 4 s during the contraction protocols, 12 s during the first 5 min of recovery, and 30 s during the final 5 min of recovery. A stacked plot of rest and exercise spectra for a typical subject is shown in Fig. 1.
Figure 1. 31P MRS spectra from the tibialis anterior muscle in a single subject.
Representative stacked plot of phosphorous spectra at rest (first spectrum, 60 s average) and throughout the six 12 s MVCs with 12 s rest intervals (4 s temporal resolution). The Pi peak split into two distinct peaks during the contractions in this subject.
Spectral analyses
Exponential multiplication corresponding to 10 Hz line broadening was applied to the free-induction decays before they were Fourier-transformed to the frequency domain. After phasing and baseline correction, the underlying broad peak due to the phosphorous in bone was removed by calculating a 5th order polynomial fit of the baseline region. Peaks corresponding to PCr, phosphomonoesters (PMEs), Pi, and γ, α and β ATP were then fitted with Lorentzian-shaped curves (NUTS software, Acorn NMR, Livermore, CA, USA) to quantify the area of each peak. When two distinct Pi peaks were observed, two Lorentzian-shaped curves were used.
Metabolic calculations
Millimolar concentrations of phosphorous metabolites were calculated assuming that [PCr] + [Cr] = 42.5 mm and resting [ATP] = 8.2 mm (Harris et al. 1974). According to the equilibrium of the creatine kinase reaction and impermeability of the cell to Pi and free creatine, we assumed that Δ[Pi] is equivalent to Δ[Cr] during rest, exercise, and recovery(Kemp & Radda, 1994). Corrections for partial saturation of the 31P-MRS peaks due to the rapid repetition time of the MR protocol were applied as previously described (Lanza et al. 2005). Intramuscular pH was calculated based on the chemical shift (σ) of Pi relative to PCr in parts per million:
| (1) |
When distinct Pi splitting was evident, the pH corresponding to each Pi pool was calculated separately as pH1 and pH2 based on the chemical shift of each peak relative to PCr. The overall muscle pH was then calculated for each spectrum as:
| (2) |
The concentration of the diprotonated form of inorganic phosphate (H2PO4−) was calculated as:
| (3) |
Adenosine diphosphate (ADP) and adenosine monophosphate (AMP) concentrations were calculated based on the equilibrium of the creatine kinase and adenylate kinase reactions, respectively, and the stoichiometry of free creatine and inorganic phosphate (Lawson & Veech, 1979):
| (4) |
| (5) |
The equilibrium constants of the creatine kinase (KCK) and adenylate kinase (KAK) reactions were corrected for the effects of pH, assuming free magnesium concentration of 1 mm (Golding et al. 1995).
ATP synthesis from oxidative phosphorylation
The rates of mitochondrial ATP synthesis (ATPOX, mm s−1) were determined from the rate of PCr recovery during the 12 s rest intervals between contractions (Kemp & Radda, 1994; Jubrias et al. 2001). This model of oxidative phosphorylation assumes that the recovery of PCr is due entirely to oxidative ATP synthesis and that anaerobic glycolysis ceases in the absence of muscle contraction (Quistorff et al. 1993; Blei et al. 1993). However, in the present study, we observed a small amount of PCr recovery during ISC, attributable to glycolytic ATP synthesis (Crowther et al. 2002; Jubrias et al. 2003). As a result, PCr recovery between contractions in the FF protocol was adjusted for the glycolytic contribution as follows:
| (6) |
Oxidative ATP synthesis was assumed to be negligible during ISC contractions since the oxygen trapped within the muscle during cuff occlusion would only be sufficient to generate ∼4 mm ATP (Harris et al. 1975; Kemp et al. 1994), an amount that would be exhausted in ∼10 s under the contraction conditions used here.
ATP synthesis from anaerobic glycolysis
Glycolytic flux (ATPGLY, mm s−1) was calculated during each 12 s MVC from exercise-induced changes in intramuscular pH and metabolites, after correcting for proton efflux, buffering capacity, the consumption of protons by the creatine kinase reaction, and a small contribution of oxidative metabolism to the proton pool (Kemp & Radda, 1994; Walter et al. 1999).
| (7) |
The terms dpH/dt and dPCr/dt are the changes in pH and PCr for each time interval, respectively. The correction factor θ accounts for the consumption of protons when PCr is hydrolysed in the creatine kinase reaction:
| (8) |
This calculation of the proton stoichiometry coefficient does not account for the possible effects of intracellular Mg2+ and K+ on proton stoichiometry, which may affect the magnitude of the coefficient (Kushmerick, 1997). However, this possible effect would not be expected to have a significant impact on the comparisons across conditions in the present study.
The correction factor m accounts for the production of protons during oxidative ATP synthesis:
| (9) |
β represents the pH buffering capacity (slykes) of the muscle, which takes into account the inherent buffering capacity of the muscle (βi) and buffering due to muscle bicarbonate (βCO2), inorganic phosphate (βPi) and phosphomonoesters (βPME), which are calculated throughout each protocol, as discussed elsewhere (Kemp & Radda, 1994; Walter et al. 1999):
| (10) |
| (11) |
where the solubility of CO2 in muscle water, S, is 1.3 mm kPa−1 and the partial pressure of CO2(PCO2) is 5 kPa.
| (12) |
| (13) |
Inherent buffering capacity was determined from changes in pH and PCr during the first 4 s of exercise when glycolysis and proton efflux are assumed to have a negligible effect on pH changes:
| (14) |
The assumption of negligible glycolysis or proton efflux during the first 4 s of contraction stems from demonstrations that the initial pH change during contraction is entirely attributable to proton consumption or strong ion changes as the creatine kinase reaction proceeds in the forward direction (Adams et al. 1990). Furthermore, at least 10 s are required to achieve full activation of glycolysis during muscle contractions (Meyer et al. 1988). Other studies in human (Conley et al. 1997) and frog (Yamada & Sugi, 1987) muscle have also reported a delay in the onset of glycolysis. Proton efflux from the cell is also assumed to be initially negligible since intracellular proton accumulation is the driving force behind the Na+/H+ antiporter and lactate–H+ cotransporter (Bendahan et al. 2003), and proton accumulation is insignificant during the first 4 s of contraction.
Proton efflux rates during exercise (νeff, mm (pH unit)−1 min−1) were estimated for each 12 s contraction, based on rates calculated during the postcontraction recovery period (Kemp & Radda, 1994; Walter et al. 1999) and assuming linear pH dependence of lactate–proton coefflux in muscle (Bangsbo et al. 1993; Newcomer et al. 1999). After the initial phase of acidosis early in recovery, pH increases linearly as a function of net proton efflux from the cell. A proportionality constant, λ, was determined from the slope of νeffversus dpH/dt during the linear portion of recovery where:
| (15) |
When these major factors contributing to the change in pH during recovery are accounted for, the remaining dpH/dt represents the rate of proton efflux. The proportionality constant, λ, was used to estimate proton efflux during the contraction protocol:
| (16) |
During ischaemia, we assumed negligible oxidative ATP synthesis and proton efflux (Kemp & Radda, 1994), thus simplifying the glycolytic rate calculation to:
| (17) |
ATP synthesis from PCr breakdown
The rate of ATP synthesis from the net breakdown of PCr via the CK reaction (ATPCK, mm s−1) was determined from the change in PCr during each contraction. Since the synthesis of ATP is stoichiometric with the hydrolysis of PCr in the creatine kinase reaction, the calculation of CK takes the simple form (Kemp & Radda, 1994):
| (18) |
Total ATPase rate
The total ATPase rate (ATPTOT mm s−1) for each contraction was determined as (Kemp & Radda, 1994; Walter et al. 1999):
| (19) |
For each contraction, ATPCK and ATPGLY were also scaled to muscle force production (FTI/FTIinitial), which allows comparisons across FF and ISC protocols by adjusting for any differences in muscle force due to fatigue. Metabolic economy (ME, N s (mm ATP)−1) was determined over the course of each contraction protocol as:
| (20) |
Exploratory studies
To provide an estimate of the intracellular oxygen content under ischaemic conditions, exploratory studies of myoglobin desaturation were performed in one representative subject. The deoxygenated form of myoglobin (dMb) was measured during ISC using 1H-MRS (Jue & Anderson, 1990). Proton spectra were acquired using a 500 μs frequency-selective Gaussian pulse, 25 ms repetition time, centred on dMb (∼80 p.p.m. downfield from water). To minimize baseline artefacts due to the water signal, a water suppression pulse was applied (500 μs Gaussian pulse, centred on water, 5 ms crusher gradient). Spectra were acquired throughout the ISC protocol and during a 10 min bout of ischaemia at rest, obtained 10 min following unoccluded recovery from the ISC protocol.
Using NUTS software, dMb data were averaged to yield 12 s time resolution. Data were zero-filled, and exponential multiplication was applied using a mixed Lorentzian-Gaussian function (−300 Hz/0.1) prior to Fourier transformation. Spectra were phased (0 and 1st order) and baseline-fitted before an automated curve-fitting procedure was applied to the dMb peak for each spectrum. All dMb values were expressed relative to the peak value obtained during the 10 min ischaemic rest period, taken to reflect complete desaturation of the muscle. We used the oxygen binding curve for myoglobin to calculate intracellular PO2, assuming a P50 for myoglobin of 3.2 Torr, where f is the fractional desaturation of myoglobin (Richardson et al. 1995):
| (21) |
On a separate day, the same subject repeated the contraction protocol following 10 min of ischaemia prior to the first contraction with 31P-MRS measures obtained continuously and analysed as described above.
Statistical analyses
Student's t test for paired data was used to compare end-exercise [PCr], [Pi], [ADP], [AMP], [PME], [ATP], [H2PO4−], pH, fatigue, and metabolic economy in FF and ISC. The non-normal distribution of CAR values necessitated non-parametric analyses using the Mann-Whitney U test. The relationships between FTI (% initial) and both H2PO4− and phosphorylation potential ([ATP]/[ADP][Pi]) were determined for each subject using linear regression analyses. Two-way (condition, time) repeated measures ANOVA was used to compare ATPGLY, ATPCK and ATPTOT between FF and ISC contractions. One-way (time) repeated measures ANOVA was used to examine ATPOX during the FF condition. The appropriate covariance structures were defined for each analysis. All analyses were performed using SAS software (Cary, NC, USA).
Results
Subject characteristics
Subjects were 27 ± 4 (mean ± s.d.) years of age. Height and mass were 171 ± 10 cm and 71 ± 17 kg, respectively. Systolic brachial and ankle blood pressures were 112 ± 11 Torr and 129 ± 14 Torr, respectively.
Force and central activation
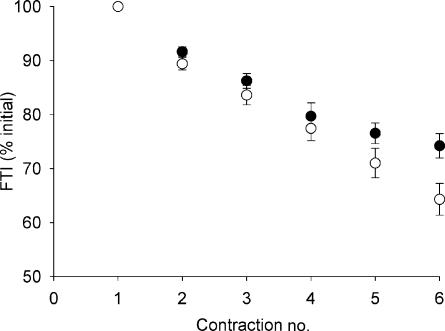
All subjects were able to fully activate the ankle dorsiflexor muscles as evidenced by CAR values of ∼1.0 in the unfatigued state (Table 1). Baseline MVC was ∼5% higher prior to ISC compared to FF (Table 1). During the FF and ISC contraction protocols, the force–time integral progressively decreased (Fig. 2), with greater fatigue occurring during ISC compared to FF (Table 1). Although some central activation failure was evident at the end of both contraction protocols (Table 1), central fatigue appeared to play a minor role in the development of muscle fatigue, as evidenced by mean CAR = 0.92 during the final contraction in both protocols.
Table 1.
Selected variables from FF and ISC protocols
| Free flow | Ischaemia | P | 95% CI | |
|---|---|---|---|---|
| Force | ||||
| MVCpre (N) | 323 ± 27 | 335 ± 27 | 0.04 | −24.7, −0.4 |
| Fatigue (FTIpost/FTIpre× 100) | 74.2 ± 2.3 | 64.3 ± 2.9 | <0.001 | 6.0, 13.8 |
| CARpre | 0.99 ± 0.005 | 1.0 ± 0.001 | 0.26 | −0.002, 0.004 |
| CARend | 0.92 ± 0.002 | 0.97 ± 0.001 | 0.11 | −0.001, 0.01 |
| Metabolites at rest | ||||
| [PCr] (mm) | 38.2 ± 0.02 | 38.0 ± 0.03 | 0.54 | −0.56, 1.02 |
| [Pi] (mm) | 4.3 ± 0.2 | 4.5 ± 0.3 | 0.71 | −0.95, 0.66 |
| pH | 7.00 ± 0.01 | 7.01 ± 0.01 | 0.25 | −0.04, 0.01 |
| H2PO4− (mm) | 1.5 ± 0.1 | 1.6 ± 0.1 | 0.89 | −0.26, 0.23 |
| PME (mm) | 3.0 ± 0.4 | 2.7 ± 0.3 | 0.75 | −1.1, 1.4 |
| ADP (mm) | 0.008 ± 0.0 | 0.008 ± 0.0 | 0.50 | −0.002, 0.001 |
| AMP (μm) | 0.007 ± 0.0 | 0.008 ± 0.0 | 0.40 | −0.004, 0.002 |
| Metabolites at end-exercise | ||||
| [PCr] (mm) | 9.9 ± 0.9 | 5.1 ± 0.8 | <0.001 | 2.7, 6.8 |
| [Pi] (mm) | 32.6 ± 0.9 | 37.4 ± 0.8 | <0.001 | −6.8, −2.7 |
| pH | 6.70 ± 0.03 | 6.60 ± 0.05 | 0.10 | −0.02, 0.22 |
| H2PO4− (mm) | 17.1 ± 0.9 | 21.4 ± 1.1 | 0.004 | −6.9, −1.6 |
| PME (mm) | 8.4 ± 0.6 | 11.4 ± 1.3 | 0.07 | −6.44, 0.29 |
| ADP (mm) | 0.15 ± 0.03 | 0.39 ± 0.09 | 0.02 | −0.40, −0.04 |
| AMP (μm) | 2.7 ± 0.9 | 23.6 ± 8.9 | 0.04 | −40.2, −1.4 |
| [ATP] (mm) | 8.5 ± 0.5 | 8.2 ± 0.6 | 0.67 | −1.1, 1.7 |
Values are means ±s.e.m. 95% confidence intervals are presented for the difference between FF and ISC.
Figure 2. Muscle fatigue of the ankle dorsiflexors.
The force–time integral during the 12 s MVCs, expressed relative to the first contraction, with (ISC, ○) and without (FF, •) local circulatory occlusion. There was more fatigue during ISC compared to FF (P < 0.001). Values are means ± s.e.m.
Phosphorous metabolites and pH
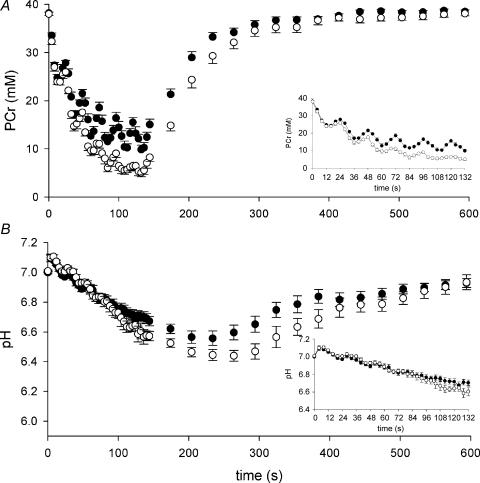
Resting [PCr], [Pi] and pH were similar prior to the two protocols (Table 1). Phosphocreatine decreased during each 12 s MVC in FF and ISC (Fig. 3A). Subsequently, PCr increased during the rest intervals between contractions, although the magnitude of this recovery was blunted during ISC compared to FF (Fig. 3A inset). At the end of the contraction protocol, PCr was depleted more in ISC compared to FF (Table 1). As expected, changes in Pi during the two protocols mirrored the changes in PCr (data not shown), and at the end of the contraction protocol Pi was higher in ISC compared to FF (Table 1). The end-exercise concentrations of ATP were similar across contraction protocols (Table 1) and did not change significantly from the assumed resting value of 8.2 mm. At the onset of FF and ISC, pH initially increased (Fig. 3B), and thereafter declined similarly during FF and ISC, although there was a trend for greater acidosis in ISC during the final contraction (Fig. 3B, Table 1). Phosphomonoesters (PMEs), consisting primarily of glucose-6-phosphate (Bloch et al. 1993), increased to a greater degree in ISC compared to FF, as did ADP and AMP (Table 1). Diprotonated Pi was similar at rest but increased to a greater extent in ISC compared to FF (Table 1).
Figure 3. Phosphocreatine and pH during contractions and recovery.
Phosphocreatine (A) decreased more during ISC (○) compared to FF (•) (P < 0.001). The inset shows the contraction period, illustrating transient PCr depletion and recovery during the contraction–relaxation cycle. Intracellular pH (B) decreased during FF and ISC, with a trend (P = 0.10) for greater acidosis during ISC. Values are means ±s.e.m.
Pathways of ATP synthesis
Figure 4 shows absolute rates (mm s−1) of ATP synthesis through ATPOX, ATPGLY and ATPCK for each contraction. We also examined ATP synthesis rates after scaling to FTI during each contraction, to account for changes in the level of muscle force production as fatigue developed. For ease of presentation, we show only the absolute ATP synthesis rates, as scaling ATP flux to muscle fatigue produced qualitatively similar results.
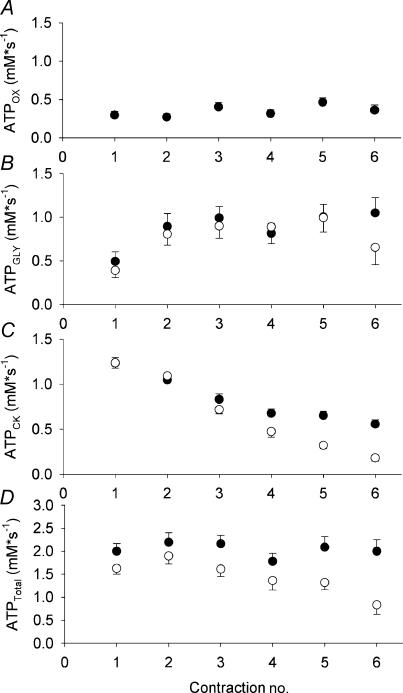
Figure 4. Rates of ATP synthesis during muscle contractions.
ATP production from oxidative phosphorylation (ATPOX, A) was similar across the 6 contractions throughout FF (P = 0.12), and assumed to be negligible during ISC. Glycolytic flux (ATPGLY, B) was similar during FF (•) and ISC (○) (P = 0.22). ATP production from net PCr breakdown in the creatine kinase reaction (ATPCK, C) was greater during FF than ISC (P < 0.001). Total ATP turnover (ATPTOT, D), calculated as the sum of ATPOX, ATPGLY, and ATPCK, was greater during FF compared to ISC (P < 0.001). Values are means ±s.e.m.
The rate of oxidative ATP synthesis was constant throughout the FF contraction protocol (Fig. 4A, Ptime = 0.12). Glycolytic flux was similar during FF and ISC (Pcondition = 0.22, Fig. 4B). In both conditions, ATPGLY increased over the course of the six contractions to a similar extent (Ptime < 0.001, Pcondition × time = 0.75).
The rates of ATP generated by net PCr hydrolysis were higher during FF compared to ISC contractions (Fig. 4C, Pcondition < 0.001), presumably due to the greater resynthesis of PCr during FF rest intervals. In both conditions, the contribution from ATPCK decreased as the contractions progressed (Ptime < 0.001), with the magnitude of this decline greater during ISC (Pcondition × time < 0.001).
The total ATPase rate was higher in FF compared to ISC (Fig. 4D, Pcondition < 0.001). Total ATPase rate declined in the ischaemic condition, but not to an extent that was different from the FF (Ptime = 0.02, Pcondition × time = 0.19). Metabolic economy was higher during ISC (166 ± 17 N s (mm ATP)−1) compared to FF (126 ± 14 N s (mm ATP)−1, P = 0.03).
Relationships between metabolites and fatigue
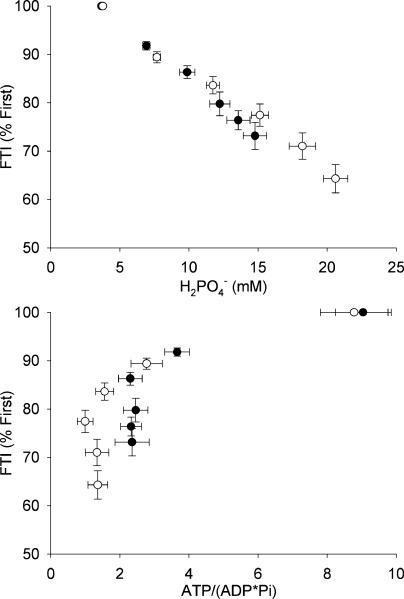
There was a strong linear relationship between FTI and intracellular H2PO4− during FF (Fig. 5A, r = 0.94 ± 0.01) and ISC (r = 0.92 ± 0.02). In contrast, the relationship between fatigue and [ATP]/[ADP][Pi] was curvilinear and shifted to the left with ischaemia, such that lower cytosolic phosphorylation potentials were observed at similar levels of fatigue in ISC compared to FF (Fig. 5B). The dissociation between phosphorylation potential and fatigue is also evidenced by the continued decrease in FTI despite a stable phosphorylation potential.
Figure 5. Relationships between muscle fatigue, pH and phosphorylation potential.
Strong linear associations between fatigue and H2PO4− were observed during FF (•) and ISC (○) (top panel); FF r = 0.93 ± 0.01, ISC r = 0.91 ± 0.03. Curvilinear relationships were evident between fatigue and intracellular phosphorylation potential during FF and ISC (bottom panel). These relationships were left-shifted during ISC compared to FF. Values are means ±s.e.m.
Exploratory studies of tissue oxygenation and energetics
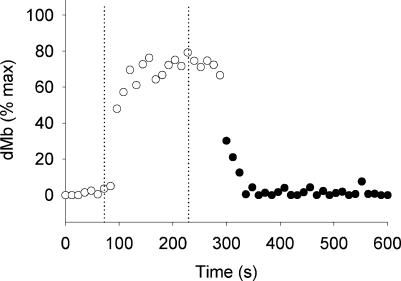
Figure 6 shows the changes in myoglobin deoxygenation during the ischaemic contraction protocol in one subject. Rapid desaturation was observed at the onset of the contractions, which reached a plateau at ∼75% of the peak desaturation observed during resting ischaemia, corresponding to an intracellular PO2 of 1.07 Torr.
Figure 6. 1H-MRS measures of deoxy-myoglobin during ischaemic contractions in one subject.
The deoxy-myoglobin signal was measured during 1 min of resting cuff occlusion (○, ischaemia), ISC protocol (contraction period defined by the dotted vertical lines), cuff occlusion for 1 min following the end of the contractions, and 10 min of free-flow recovery (•, release of cuff occlusion). Ischaemic muscle contractions resulted in myoglobin desaturation to ∼75% of the desaturation observed during 10 min of resting ischaemia.
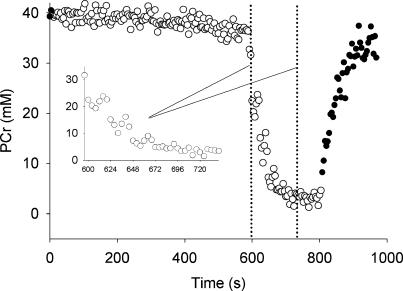
Figure 7 shows the changes in PCr during 10 min of ischaemia at rest, followed by the ISC protocol in one subject. Phosphocreatine showed a slight downward drift during the ischaemic rest period. Thereafter, PCr decreased rapidly during the six ischaemic contractions. Notably, we observed some recovery of PCr during the ischaemic rest intervals between contractions (Fig. 7, inset). This recovery was less evident during the final stages of the contraction protocol, and no PCr recovery was observed following the final contraction until blood flow was restored.
Figure 7. 31P-MRS measures of phosphocreatine during prolonged ischaemia followed by ischaemic contractions in one subject.
PCr decreased slightly after ∼8 min of resting ischaemia (○, ischaemia). Muscle contractions were initiated after 10 min of ischaemia, resulting in a marked decrease in PCr (the start and end of the contraction periods are indicated by the dotted lines). Inset shows PCr during 6 MVC/rest intervals. PCr recovery was observed following the first 3 contractions. No recovery of PCr was evident when ischaemia was maintained for 1 min after the final MVC, but PCr recovered quickly when blood flow was restored (•, release of cuff occlusion).
Discussion
Our results indicate that, during ischaemic contractions when oxidative phosphorylation is no longer a viable source of ATP, the balance between ATP supply and demand is met in vivo not by increasing glycolytic flux, but by decreasing ATP demand. Furthermore, the present results suggest that the reduced demand for ATP during ISC is accomplished by a combination of increased metabolic economy and decreased muscle force production as fatigue develops. Under both FF and ISC conditions, fatigue was related to intracellular H2PO4− rather than decreased ATP turnover mediated by reductions in the phosphorylation potential of the muscle.
Force and central activation
The force–time integral decreased to a greater extent during ischaemic compared to free-flow contractions. These data support the work of other investigators who have shown that skeletal muscle fatigues more during circulatory occlusion than under free-flow conditions (Harris et al. 1975; Greenhaff et al. 1993; Kemp et al. 1994; Russ & Kent-Braun, 2003). Further, we found that central activation was unimpaired at baseline and maintained equally well (CAR = 0.92) during both 132 s contraction protocols, indicating that the volume of active muscle was largely conserved throughout both protocols. These data support the assumption that the observed metabolic changes were representative of the entire muscle.
Anaerobic glycolysis
The magnitude of the glycolytic rates measured in the present study (0.40–1.05 mm s−1) is similar to reports from other investigators who have used 31P-MRS to quantify glycolytic flux in vivo (0.1–3.0 mm s−1) (Kemp et al. 1994; Walter et al. 1999). Contrary to our hypothesis, when blood flow to the muscle was eliminated, glycolytic ATP production was unchanged compared to free-flow contractions. This result conflicts with previous reports of increased glycolytic flux when oxygen delivery is limited (Linnarsson et al. 1974; King et al. 1987; Greenhaff et al. 1993; Kemp et al. 1994; Timmons et al. 1996; Greiner et al. 2005). One explanation for these disparate results may be the intensity of the muscle contractions during which glycolytic flux was measured. Since some previous studies employed submaximal contractions (Kemp et al. 1994; Greiner et al. 2005), it is possible that the ischaemia-induced increase in glycolytic flux in those studies was related to altered recruitment during ischaemia (Takarada et al. 2000). That is, glycolytic, Type II fibres may have been recruited more during ischaemia, which could produce higher overall glycolytic rates as a result. Consistent with this notion, muscle lactate concentration has been shown to be related to oxygen availability at submaximal but not maximal workloads (Linnarsson et al. 1974). The present study used maximal intensity muscle contractions, which recruit the entire population of muscle fibres, and presumably would not be affected by changes in muscle fibre recruitment between the FF and ISC protocols.
Greenhaff et al. (1993) overcame the issue of muscle recruitment effects by examining muscle energy metabolism in response to maximal electrical stimulation of human quadriceps muscles. Interestingly, these investigators reported similar glycogenolytic rates in type II fibres during contractions with and without ischaemia, assessed through biopsies before and immediately after stimulation. However, type I fibres exhibited substantial increases in glycolytic flux during ISC compared to FF. The authors suggested that type II fibres are functioning close to Vmax for glycogenolysis during maximal contractions with intact circulation, unlike type I fibres, which may increase glycolytic flux when cellular concentrations of known activators of glycogenolysis are increased during ischaemia. It is somewhat surprising, then, that the present study of the tibialis anterior muscle (∼76% type I fibres; Jakobsson et al. 1988) failed to demonstrate a similar result. While we cannot explain the discrepancy between our report and that of Greenhaff et al. (1993) our observations are consistent with a previous study in the same muscle group. Conley et al. (1998) performed maximal stimulation of the tibialis anterior muscle while using 31P-MRS to assess glycolytic flux in a similar manner to that of the present study (Conley et al. 1998). Thus, it appears possible that the conflicting results of Greenhaff et al. (1993) may be related to differences in the muscle group studied, the manner in which the muscle was activated (voluntary MVC, supramaximal nerve stimulation, maximal motor point stimulation) or the methods used to quantify glycolytic flux.
The metabolic response of ischaemic muscle also appears to be sensitive to the manner in which oxygen supply is limited. Gutierrez et al. (1988) reported that ischaemia and hypoxia affected skeletal muscle bioenergetics in different ways. Hypoxia of rabbit hindlimb was accomplished by decreasing the PO2 of arterial blood to ∼5 Torr, while ischaemia was accomplished by eliminating blood flow by arterial occlusion. Blood lactate and intracellular pH changes were similar in control and ischaemic conditions. However, hypoxia resulted in a much greater change in blood lactate and pH from resting values compared to ischaemia. The authors highlight the possibility that, unlike complete ischaemia, hypoxia allows delivery of substrate and efflux of metabolic by-products from the muscle that may otherwise inhibit glycolytic flux. This possibility was confirmed in contracting canine gastrocnemius muscle during partial ischaemia (∼30% reduction in blood flow) and free-flow hypoxia (11% fraction of inspired O2) (Stainsby et al. 1990). When ischaemia was induced at the onset of muscle contractions, the net lactic acid output from the muscle did not increase compared to controls. When free-flow hypoxia was introduced at the onset of contractions, net lactic acid output was greater than controls. The results suggest that anaerobic glycolysis was able to compensate for decreased 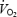 during hypoxia, but not ischaemia, which likely explains why mechanical performance declined more during ischaemia compared to hypoxia (Stainsby et al. 1990). These studies provide an attractive explanation for the discordance between the results of the present study and those of others who have limited muscle oxygenation by free-flow hypoxia or partial ischaemia (King et al. 1987; Gutierrez et al. 1988; Stainsby et al. 1990; Timmons et al. 1996).
during hypoxia, but not ischaemia, which likely explains why mechanical performance declined more during ischaemia compared to hypoxia (Stainsby et al. 1990). These studies provide an attractive explanation for the discordance between the results of the present study and those of others who have limited muscle oxygenation by free-flow hypoxia or partial ischaemia (King et al. 1987; Gutierrez et al. 1988; Stainsby et al. 1990; Timmons et al. 1996).
Our results demonstrate that during maximal voluntary contractions, glycolytic flux measured in vivo is similar during free-flow and ischaemia. We anticipated that glycolytic flux would increase during ISC compared to FF due to the suppression of oxidative ATP synthesis and continued high demand for ATP. It is reasonable to expect accumulation of pyruvate and NADH when mitochondrial oxygen availability is limited. Since NADH and pyruvate are both substrates for the lactate dehydrogenase reaction, lactate production could increase because of mass action effects. Glycolytic flux may also be higher during ischaemia due to greater accumulation of Pi, which is believed to be a potent activator of glycogen phosphorylase (Chasiotis et al. 1982). Other putative glycolytic regulators that may be relatively higher during ischaemic compared with free-flow contractions include AMP, which regulates the conversion of phosphorylase b to a through the combined effects of calcium on the activity of phosphorylase kinase (Greenhaff et al. 1993). Indeed, we found that Pi, AMP and ADP were higher during ISC compared to FF (Table 1). The similarity of glycolytic flux under both conditions despite elevated levels of these metabolites suggests that glycolytic flux was already near Vmax during FF contractions, as suggested by Greenhaff et al. (1991, 1993) in type II fibres.
The high demand for ATP during the maximal intensity muscle contractions employed in the present study necessitated substantial glycolytic flux to supply ample ATP, even during FF contractions. Furthermore, voluntary contractions of this muscle group above 60% MVC have recently been shown to generate sufficient intramuscular pressure to compress the vasculature and fully occlude muscle perfusion (Wigmore et al. 2004). Therefore, it is likely that glycolytic flux increased in FF during the 12 s MVCs due to transient limitations in muscle oxygenation. Alternatively, glycolytic flux during ISC may have been limited, not by an upper limit to glycolytic flux, but by inhibition of rate-limiting steps in glycolytic reactions during ISC. Intracellular proton accumulation will eventually inhibit phosphofructokinase, thus limiting carbon flux through the distal reactions in glycolysis (Chasiotis et al. 1982). We observed a trend for greater acidosis during ISC compared to FF, suggesting that proton accumulation may have begun to limit glycolytic flux more during ISC than FF. We also observed a trend toward greater accumulation of phosphomonoesters, consisting principally of glucose-6-phosphate, during ISC compared to FF. This result suggests that proton accumulation during ISC may have limited glycolytic flux through inhibition of phosphofructokinase, with subsequent accumulation of glucose-6-phosphate.
Oxidative phosphorylation
The rates of oxidative ATP synthesis determined from the initial postcontraction PCr recovery during FF (0.2–0.5 mm s−1) were similar to rates reported during voluntary contractions in previous studies (0.1–0.7 mm s−1) (Conley et al. 1998; Kemp et al. 1994; Lanza et al. 2005). Interestingly, oxidative flux remained constant with each successive contraction, despite increases in several purported activators of mitochondrial respiration. The rates of oxidative ATP synthesis may have been limited by oxygen availability, even during the FF protocol. As mentioned earlier, ankle dorsiflexion above 60% MVC results in vascular occlusion due to high intramuscular pressure (Wigmore et al. 2004), which could have imposed an upper limit on the increase in oxidative phosphorylation during this intermittent MVC protocol.
The model of PCr recovery for estimates of oxidative phosphorylation is well-established (Kemp & Radda, 1994; Jubrias et al. 2001). However, we observed a small amount of PCr resynthesis during each 12 s rest interval during ischaemia following the first 3–4 contractions (Fig. 3A). Given that the cuff pressure (220 Torr) was significantly higher than systolic blood pressure measured at rest in the lower leg of these subjects, and mean arterial pressure increases by ∼30 Torr from rest to intense contractions of the leg musculature (Andersen & Saltin, 1985), it is unlikely that this small amount of PCr recovery was due to oxygen leaking into the muscle. Further support for this notion comes from the observation that the end-exercise PCr level does not recover until blood flow is restored as demonstrated in the present study (Fig. 7) and by others (Quistorff et al. 1993; Blei et al. 1993).
Alternatively, it is possible that a sufficient reservoir of oxygen bound to trapped haemoglobin and myoglobin could sustain oxidative ATP synthesis at low rates during blood flow occlusion. To address this possibility, we measured deoxy-myoglobin during the ischaemic contraction protocol in a representative subject. Myoglobin desaturated by ∼75%, corresponding to an intracellular PO2 of 1.07 Torr, which appears to be above the PO2 at which oxidative function will be limited in tissue at > 50% maximal ATP flux (Connett et al. 1990). An earlier study using near-infrared spectroscopy also showed that muscle deoxygenated less during a period of ischaemia with interspersed contractions than during an equivalent period of ischaemic rest (De Blasi et al. 1992). The incomplete desaturation of myoglobin observed during ischaemic contractions may be the result of the inhibition of oxidative ATP production by the metabolic by-products of anaerobic glycolysis (Conley et al. 2001) or the competition of oxygen and nitric oxide for binding sites on cytochrome oxidase in the mitochondria (Cleeter et al. 1994). Despite incomplete desaturation of intracellular oxygen, oxidative ATP synthesis was unlikely to be responsible for the recovery of PCr between contractions during ISC, particularly since trapped oxygen within the ischaemic muscle would only be sufficient to generate ∼4 mm of ATP through oxidative phosphorylation (Harris et al. 1975; Kemp et al. 1994).
To further confirm that PCr recovery during ISC could not be due to residual oxygen trapped within the ischaemic muscle, one representative subject performed the six intermittent contractions after a 10 min period of ischaemia at rest, which was used to completely deoxygenate the muscle. We observed a similar pattern of PCr resynthesis between ischaemic contractions as observed when only 30 s of ischaemia preceded the contraction protocol (Fig. 7). These data make it difficult to ignore the possibility that the PCr recovery observed during ISC was unrelated to oxidative ATP production.
Our demonstration of PCr recovery during ischaemic rest intervals is in contrast to several previous reports that PCr is not resynthesized during ischaemic recovery (Harris et al. 1975; Blei et al. 1993; Quistorff et al. 1993). These investigators have concluded that despite high levels of Pi and AMP, which are activators of phosphofructokinase and glycogen phosphorylase, anaerobic glycolysis ceases in the absence of muscle contraction, presumably due to the absence of calcium release into the cytosol by the sarcoplasmic reticulum (Connett & Sahlin, 1996). In contrast, others have reported that glycolytic flux continues for several seconds following the cessation of muscle activity (Crowther et al. 2002), suggesting that a small amount of PCr resynthesis between contractions during ISC could be provided by glycolysis. In the present study, the signal-to-noise associated with the 4.0 telsa field strength allowed us to examine PCr changes with a 4 s time resolution, permitting observation of changes in PCr during the first few seconds of recovery. Our PCr data during ISC provide new evidence of glycolytic ATP synthesis in resting muscle in vivo. Further study is necessary to clarify the factors regulating glycolytic flux in the absence of muscle contraction.
Phosphocreatine hydrolysis
The ATP derived from the net breakdown of PCr was greatest during the early phases of both contraction protocols. As the contractions progressed, the contribution of this pathway to ATP synthesis decreased, in agreement with previous studies in the ankle dorsiflexors (Lanza et al. 2005) and plantarflexors (Walter et al. 1999). This decrease was more pronounced during ISC such that FF contractions derived relatively more ATP from net PCr breakdown. This finding is undoubtedly a result of greater PCr resynthesis during rest intervals in FF compared to ISC, leading to higher PCr concentrations at the onset of subsequent contractions (Fig. 3A).
Total ATPase rate
Overall ATPase rates were lower during ISC compared to FF (Fig. 4D). Since the rate of ATP consumption depends on muscle force production, the lower ATPase rate during ISC could reflect the greater decline in muscle force during the ischaemic contractions. However, the lower ATPase rate during ISC compared to FF persisted even after we accounted for differences in muscle force production by normalizing to the degree of muscle fatigue, suggesting that the declines in muscle contractility during ISC could not entirely explain the decline in ATPase rates. Indeed, metabolic economy was higher during ISC compared to FF, in accordance with work of other investigators who have observed greater metabolic efficiency during anaerobic than aerobic ATP synthesis (Krustrup et al. 2003). These investigators report that heat production in exercising muscle is 60% lower when blood flow is occluded in comparison to free-flow exercise at the same workload, in accordance with early experiments in vitro (Curtin & Woledge, 1978). Intracellular calcium transport into the mitochondria has been implicated as a mechanism for this reduced efficiency, by causing uncoupling of oxidation and phosphorylation (Halestrap et al. 1993). The increased metabolic economy observed during ISC is clearly a mechanism by which the balance between ATP supply and demand is balanced in ischaemic muscle.
Relationships between metabolites and fatigue
To address the causes of greater fatigue during ISC compared to FF, we examined the relationships between fatigue, intracellular H2PO4−, and the phosphorylation potential of the muscle. The linear association between fatigue and intracellular H2PO4− (Fig. 5) suggests that fatigue was related to the synergistic inhibitory effects of protons and Pi produced during glycolytic ATP turnover, perhaps leading to greater fatigability of ischaemic muscle. While glycolytic flux was similar during FF and ISC and would be expected to elicit a similar degree of acidosis under both conditions, proton efflux was presumably prevented during ISC, which could explain the trend for greater acidosis, and therefore greater accumulation of H2PO4− and fatigue during ISC compared to FF.
To test the hypothesis that the energetic state of the muscle will regulate force production, we examined the relationship between fatigue and intracellular phosphorylation potential, which is purported to modify the demand of the ATPases (Arthur et al. 1992). The phosphorylation potential of the muscle, determined as [ATP]/[ADP][Pi], decreases when oxidative and glycolytic pathways cannot satisfy the demand for ATP. We observed a non-linear relationship between force and energy state: a plateau in the phosphorylation potential was seen as muscle force production continued to decrease. Further, there was a leftward shift in this relationship with ISC such that the phosphorylation potential was lower during ISC than FF at the same degree of muscle fatigue. Together, these analyses suggest that fatigue was primarily the result of inhibitory by-products of glycolytic ATP turnover, rather than lower ATP turnover due to a distinct effect of decreased phosphorylation potential in the muscle.
In summary, we show that glycolytic flux in maximally contracting human skeletal muscle in vivo is similar under free-flow and ischaemic conditions. We observed no net breakdown of muscle ATP during FF or ISC, suggesting that the balance of ATP supply and demand during several minutes of muscular work was maintained even during ischaemia, where oxidative ATP synthesis is negligible. This energetic balance was maintained through lower demand for ATP during ISC, accomplished by a combination of increased metabolic economy and decreased muscle force production as muscle fatigue developed.
Acknowledgments
We thank Douglas Rothman, PhD, John Buonaccorsi, PhD, Peter Brown, Mark Abildgaard, Linda Chung, Damien Callahan, Mike Tevald, PT, PhD, David Russ, PT, PhD, Steve Foulis, and Ryan Larsen, MS, for support, thoughtful discussions, and assistance in various aspects of the study. Special thanks to all of the subjects for their participation. This research was supported by NIH/NIA Grant R01 A621094 and The American College of Sport Medicine Doctoral Student Research Grant.
References
- Adams GR, Foley JM, Meyer RA. Muscle buffer capacity estimated from pH changes during rest-to-work transitions. J Appl Physiol. 1990;69:968–972. doi: 10.1152/jappl.1990.69.3.968. [DOI] [PubMed] [Google Scholar]
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur PG, Hogan MC, Bebout DE, Wagner PD, Hochachka PW. Modeling the effects of hypoxia on ATP turnover in exercising muscle. J Appl Physiol. 1992;73:737–742. doi: 10.1152/jappl.1992.73.2.737. [DOI] [PubMed] [Google Scholar]
- Bangsbo J, Johansen L, Graham T, Saltin B. Lactate and H+ effluxes from human skeletal muscles during intense, dynamic exercise. J Physiol. 1993;462:115–133. doi: 10.1113/jphysiol.1993.sp019546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendahan D, Kemp GJ, Roussel M, Fur YL, Cozzone PJ. ATP synthesis and proton handling in muscle during short periods of exercise and subsequent recovery. J Appl Physiol. 2003;94:2391–2397. doi: 10.1152/japplphysiol.00589.2002. [DOI] [PubMed] [Google Scholar]
- Blei ML, Conley KE, Kushmerick MJ. Separate measures of ATP utilization and recovery in human skeletal muscle. J Physiol. 1993;465:203–222. doi: 10.1113/jphysiol.1993.sp019673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch G, Chase JR, Avison MJ, Shulman RG. In vivo 31P NMR measurement of glucose-6-phosphate in the rat muscle after exercise. Magn Reson Med. 1993;30:347–350. doi: 10.1002/mrm.1910300311. [DOI] [PubMed] [Google Scholar]
- Chance B, Leigh JS, Clark BJ, Maris J, Kent J, Nioka S, Smith D. Control of oxidative metabolism and oxygen delivery in human skeletal muscle: a steady-state analysis of the work/energy cost transfer function. Proc Natl Acad Sci U S A. 1985;82:8384–8388. doi: 10.1073/pnas.82.24.8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasiotis D, Sahlin K, Hultman E. Regulation of glycogenolysis in human muscle at rest and during exercise. J Appl Physiol. 1982;53:708–715. doi: 10.1152/jappl.1982.53.3.708. [DOI] [PubMed] [Google Scholar]
- Cleeter MW, Cooper JM, Darley-Usmar VM, Moncada S, Schapira AH. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 1994;345:50–54. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- Conley KE, Blei ML, Richards TL, Kushmerick MJ, Jubrias SA. Activation of glycolysis in human muscle in vivo. Am J Physiol Cell Physiol. 1997;273:C306–C315. doi: 10.1152/ajpcell.1997.273.1.C306. [DOI] [PubMed] [Google Scholar]
- Conley KE, Kemper WF, Crowther GJ. Limits to sustainable muscle performance: interaction between glycolysis and oxidative phosphorylation. J Exp Biol. 2001;204:3189–3194. doi: 10.1242/jeb.204.18.3189. [DOI] [PubMed] [Google Scholar]
- Conley KE, Kushmerick MJ, Jubrias SA. Glycolysis is independent of oxygenation state in stimulated human skeletal muscle in vivo. J Physiol. 1998;511:935–945. doi: 10.1111/j.1469-7793.1998.935bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
Connett RJ, Honig CR, Gayeski TE, Brooks GA. Defining hypoxia: a systems view of
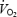 , glycolysis, energetics, and intracellular PO2. J Appl Physiol. 1990;68:833–842. doi: 10.1152/jappl.1990.68.3.833. [DOI] [PubMed] [Google Scholar]
, glycolysis, energetics, and intracellular PO2. J Appl Physiol. 1990;68:833–842. doi: 10.1152/jappl.1990.68.3.833. [DOI] [PubMed] [Google Scholar] - Connett RJ, Sahlin K. Control of glycolysis and glycogen metabolism. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 870–911. [Google Scholar]
- Crowther GJ, Kemper WF, Carey MF, Conley KE. Control of glycolysis in contracting skeletal muscle. II. Turning it off. Am J Physiol Endocrinol Metab. 2002;282:E74–E79. doi: 10.1152/ajpendo.2002.282.1.E74. [DOI] [PubMed] [Google Scholar]
- Curtin NA, Woledge RC. Energy changes and muscular contraction. Physiol Rev. 1978;58:690–761. doi: 10.1152/physrev.1978.58.3.690. [DOI] [PubMed] [Google Scholar]
- Dawson MJ, Gadian DG, Wilkie DR. Contraction and recovery of living muscles studies by 31P nuclear magnetic resonance. J Physiol. 1977;267:703–735. doi: 10.1113/jphysiol.1977.sp011835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MJ, Gadian DG, Wilkie DR. Muscular fatigue investigated by phosphorus nuclear magnetic resonance. Nature. 1978;274:861–866. doi: 10.1038/274861a0. [DOI] [PubMed] [Google Scholar]
- De Blasi RA, Cope M, Ferrari M. Oxygen consumption of human skeletal muscle by near infrared spectroscopy during tourniquet-induced ischemia in maximal voluntary contraction. Adv Exp Med Biol. 1992;317:771–777. doi: 10.1007/978-1-4615-3428-0_94. [DOI] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J Physiol. 1978;276:233–255. doi: 10.1113/jphysiol.1978.sp012231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding EM, Teague WE, Jr, Dobson GP. Adjustment of K′ to varying pH and pMg for the creatine kinase, adenylate kinase and ATP hydrolysis equilibria permitting quantitative bioenergetic assessment. J Exp Biol. 1995;198:1775–1782. doi: 10.1242/jeb.198.8.1775. [DOI] [PubMed] [Google Scholar]
- Greenhaff PL, Ren JM, Soderlund K, Hultman E. Energy metabolism in single human muscle fibers during contraction without and with epinephrine infusion. Am J Physiol. 1991;260:E713–E718. doi: 10.1152/ajpendo.1991.260.5.E713. [DOI] [PubMed] [Google Scholar]
- Greenhaff PL, Soderlund K, Ren JM, Hultman E. Energy metabolism in single human muscle fibres during intermittent contraction with occluded circulation. J Physiol. 1993;460:443–453. doi: 10.1113/jphysiol.1993.sp019480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner A, Esterhammer R, Pilav S, Arnold W, Santner W, Neuhauser B, Fraedrich G, Jaschke WR, Schocke MF. High-energy phosphate metabolism in the calf muscle during moderate isotonic exercise under different degrees of cuff compression: a phosphorus 31 magnetic resonance spectroscopy study. J Vasc Surg. 2005;42:259–267. doi: 10.1016/j.jvs.2005.04.042. [DOI] [PubMed] [Google Scholar]
- Gutierrez G, Pohil RJ, Andry JM, Strong R, Narayana P. Bioenergetics of rabbit skeletal muscle during hypoxemia and ischemia. J Appl Physiol. 1988;65:608–616. doi: 10.1152/jappl.1988.65.2.608. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Griffiths EJ, Connern CP. Mitochondrial calcium handling and oxidative stress. Biochem Soc Trans. 1993;21:353–358. doi: 10.1042/bst0210353. [DOI] [PubMed] [Google Scholar]
- Harris RC, Hultman E, Kaijser L, Nordesjo LO. The effect of circulatory occlusion on isometric exercise capacity and energy metabolism of the quadriceps muscle in man. Scand J Clin Lab Invest. 1975;35:87–95. [PubMed] [Google Scholar]
- Harris RC, Hultman E, Nordesjo LO. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest. 1974;33:109–120. [PubMed] [Google Scholar]
- Hogan MC, Gladden LB, Grassi B, Stary CM, Samaja M. Bioenergetics of contracting skeletal muscle after partial reduction of blood flow. J Appl Physiol. 1998;84:1882–1888. doi: 10.1152/jappl.1998.84.6.1882. [DOI] [PubMed] [Google Scholar]
- Hogan MC, Kurdak SS, Arthur PG. Effect of gradual reduction in O2 delivery on intracellular homeostasis in contracting skeletal muscle. J Appl Physiol. 1996;80:1313–1321. doi: 10.1152/jappl.1996.80.4.1313. [DOI] [PubMed] [Google Scholar]
- Hogan MC, Richardson RS, Haseler LJ. Human muscle performance and PCr hydrolysis with varied inspired oxygen fractions: a 31P-MRS study. J Appl Physiol. 1999;86:1367–1373. doi: 10.1152/jappl.1999.86.4.1367. [DOI] [PubMed] [Google Scholar]
- Hultman E, Sjoholm H. Energy metabolism and contraction force of human skeletal muscle in situ during electrical stimulation. J Physiol. 1983;345:525–532. doi: 10.1113/jphysiol.1983.sp014994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson F, Borg K, Edstrom L, Grimby L. Use of motor units in relation to muscle fiber type and size in man. Muscle Nerve. 1988;11:1211–1218. doi: 10.1002/mus.880111205. [DOI] [PubMed] [Google Scholar]
- Jubrias SA, Crowther GJ, Shankland EG, Gronka RK, Conley KE. Acidosis inhibits oxidative phosphorylation in contracting human skeletal muscle in vivo. J Physiol. 2003;553:589–599. doi: 10.1113/jphysiol.2003.045872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubrias SA, Esselman PC, Price LB, Cress ME, Conley KE. Large energetic adaptations of elderly muscle to resistance and endurance training. J Appl Physiol. 2001;90:1663–1670. doi: 10.1152/jappl.2001.90.5.1663. [DOI] [PubMed] [Google Scholar]
- Jue T, Anderson S. 1H NMR observation of tissue myoglobin: an indicator of cellular oxygenation in vivo. Magn Reson Med. 1990;13:524–528. doi: 10.1002/mrm.1910130322. [DOI] [PubMed] [Google Scholar]
- Kemp GJ, Radda GK. Quantitative interpretation of bioenergetic data from 31P and 1H magnetic resonance spectroscopic studies of skeletal muscle: an analytical review. Magn Reson Q. 1994;10:43–63. [PubMed] [Google Scholar]
- Kemp GJ, Thompson CH, Barnes PR, Radda GK. Comparisons of ATP turnover in human muscle during ischemic and aerobic exercise using 31P magnetic resonance spectroscopy. Magn Reson Med. 1994;31:248–258. doi: 10.1002/mrm.1910310303. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA, Le Blanc R. Quantitation of central activation failure during maximal voluntary contractions in humans. Muscle Nerve. 1996;19:861–869. doi: 10.1002/(SICI)1097-4598(199607)19:7<861::AID-MUS8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- King CE, Dodd SL, Cain SM. Muscle O2 deficit during hypoxia and two levels of O2 demand. J Appl Physiol. 1987;62:1384–1391. doi: 10.1152/jappl.1987.62.4.1384. [DOI] [PubMed] [Google Scholar]
- Krustrup P, Ferguson RA, Kjaer M, Bangsbo J. ATP and heat production in human skeletal muscle during dynamic exercise: higher efficiency of anaerobic than aerobic ATP resynthesis. J Physiol. 2003;549:255–269. doi: 10.1113/jphysiol.2002.035089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushmerick MJ. Multiple equilibria of cations with metabolites in muscle bioenergetics. Am J Physiol Cell Physiol. 1997;272:C1739–C1747. doi: 10.1152/ajpcell.1997.272.5.C1739. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Befroy DE, Kent-Braun JA. Age-related changes in ATP-producing pathways in human skeletal muscle in vivo. J Appl Physiol. 2005;99:1736–1744. doi: 10.1152/japplphysiol.00566.2005. [DOI] [PubMed] [Google Scholar]
- Lawson JW, Veech RL. Effects of pH and free Mg2+ on the Keq of the creatine kinase reaction and other phosphate hydrolyses and phosphate transfer reactions. J Biol Chem. 1979;254:6528–6537. [PubMed] [Google Scholar]
- Linnarsson D, Karlsson J, Fagraeus L, Saltin B. Muscle metabolites and oxygen deficit with exercise in hypoxia and hyperoxia. J Appl Physiol. 1974;36:399–402. doi: 10.1152/jappl.1974.36.4.399. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Fisher MJ, Nelson SJ, Brown TR. Evaluation of manual methods for integration of in vivo phosphorus NMR spectra. NMR Biomed. 1988;1:131–135. doi: 10.1002/nbm.1940010306. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Foley JM. Cellular processes integrating the metabolic response to exercise. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 841–869. [Google Scholar]
- Miller RG, Boska MD, Moussavi RS, Carson PJ, Weiner MW. 31P nuclear magnetic resonance studies of high energy phosphates and pH in human muscle fatigue. Comparison of aerobic and anaerobic exercise. J Clin Invest. 1988;81:1190–1196. doi: 10.1172/JCI113434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer BR, Boska MD, Hetherington HP. Non-Pi buffer capacity and initial phosphocreatine breakdown and resynthesis kinetics of human gastrocnemius/soleus muscle groups using 0.5 s time-resolved 31P MRS at 4.1 T. NMR Biomed. 1999;12:545–551. doi: 10.1002/(sici)1099-1492(199912)12:8<545::aid-nbm595>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Nosek TM, Fender KY, Godt RE. It is diprotonated inorganic phosphate that depresses force in skinned skeletal muscle fibers. Science. 1987;236:191–193. doi: 10.1126/science.3563496. [DOI] [PubMed] [Google Scholar]
- Quistorff B, Johansen L, Sahlin K. Absence of phosphocreatine resynthesis in human calf muscle during ischaemic recovery. Biochem J. 1993;291:681–686. doi: 10.1042/bj2910681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RS, Noyszewski EA, Kendrick KF, Leigh JS, Wagner PD. Myoglobin O2 desaturation during exercise. Evidence of limited O2 transport. J Clin Invest. 1995;96:1916–1926. doi: 10.1172/JCI118237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ DW, Kent-Braun JA. Sex differences in human skeletal muscle fatigue are eliminated under ischemic conditions. J Appl Physiol. 2003;94:2414–2422. doi: 10.1152/japplphysiol.01145.2002. [DOI] [PubMed] [Google Scholar]
-
Stainsby WN, Brechue WF, O'Drobinak DM, Barclay JK. Effects of ischemic and hypoxic hypoxia on
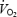 and lactic acid output during tetanic contractions. J Appl Physiol. 1990;68:574–579. doi: 10.1152/jappl.1990.68.2.574. [DOI] [PubMed] [Google Scholar]
and lactic acid output during tetanic contractions. J Appl Physiol. 1990;68:574–579. doi: 10.1152/jappl.1990.68.2.574. [DOI] [PubMed] [Google Scholar] - Takarada Y, Takazawa H, Sato Y, Takebayashi S, Tanaka Y, Ishii N. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol. 2000;88:2097–2106. doi: 10.1152/jappl.2000.88.6.2097. [DOI] [PubMed] [Google Scholar]
- Timmons JA, Poucher SM, Constantin-Teodosiu D, Worrall V, MacDonald IA, Greenhaff PL. Metabolic responses of canine gracilis muscle during contraction with partial ischemia. Am J Physiol. 1996;270:E400–E406. doi: 10.1152/ajpendo.1996.270.3.E400. [DOI] [PubMed] [Google Scholar]
- Van der Laarse WJ, Elzinga G, Woledge RC. Energetics at the single cell level. News Physiol Sci. 1989;4:91–93. [Google Scholar]
- Walter G, Vandenborne K, Elliott M, Leigh JS. In vivo ATP synthesis rates in single human muscles during high intensity exercise. J Physiol. 1999;519:901–910. doi: 10.1111/j.1469-7793.1999.0901n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigmore DM, Damon BM, Pober DM, Kent-Braun JA. MRI measures of perfusion-related changes in human skeletal muscle during progressive contractions. J Appl Physiol. 2004;97:2385–2394. doi: 10.1152/japplphysiol.01390.2003. [DOI] [PubMed] [Google Scholar]
- Yamada T, Sugi H. 31P-NMR study of the regulation of glycogenolysis in living skeletal muscle. Biochim Biophys Acta. 1987;931:170–174. doi: 10.1016/0167-4889(87)90203-5. [DOI] [PubMed] [Google Scholar]