Abstract
A central principle of signal transduction is the appropriate control of the process so that relevant signals can be detected with fine spatial and temporal resolution. In the case of lipid-mediated signaling, organization and metabolism of specific lipid mediators is an important aspect of such control. Herein, we review the emerging evidence regarding the roles of Sec14-like phosphatidylinositol transfer proteins (PITPs) in the action of intracellular signaling networks; particularly as these relate to membrane trafficking. Finally, we explore developing ideas regarding how Sec14-like PITPs execute biological function. As Sec14-like proteins define a protein superfamily with diverse lipid (or lipophile) binding capabilities, it is likely these under-investigated proteins will be ultimately demonstrated as a ubiquitously important set of biological regulators whose functions influence a large territory in the signaling landscape of eukaryotic cells.
Keywords: PITP, membrane trafficking, Sec14p, PITPs, lipids, signaling, genetics, polarized membrane growth
Introduction
PITPs are characterised by their ability to mediate the energy independent transfer of PtdIns or PtdCho monomers between membrane bilayers in vitro [1, 2]. The major PITP in the yeast Saccharomyces cerevisiae, encoded by the essential SEC14 gene, is a peripheral membrane protein of the Golgi apparatus where its function is necessary for membrane trafficking from the TGN subcompartment of this organelle [3]. Bioinformatic analyses identify Sec14-like proteins as uniquely eukaryotic proteins that are found in all eukaryotic genomes sequenced to date, and are abundant (>500 members) [4]. While this abundance defines a Sec14-protein superfamily, there is remarkably little information regarding their precise physiological functions within cells. Moreover, there is essentially no information regarding the biochemistry of how Sec14p, Sec14-like proteins, and PITPs in general execute phospholipids binding/exchange reactions. It is remarkable that a soluble protein is able to efficiently extract a phospholipid molecule from a membrane bilayer without the need ATP hydrolysis or the action of other cofactors. How release of bound phospholipid is regulated is also mysterious. Thus, the PITP phospholipid binding/exchange cycle promises to be an intriguing study in how conformational dynamics of a lipid binding protein couple to an unusual ligand binding reaction.
Herein, we summarize what is known regarding the biological functions of Sec14-like proteins that themselves constitute the Sec14 superfamily. As indicated above, this superfamily consists of greater than 500 members, of which the yeast Sec14p is the prototype, and is limited to the Eukaryota [4]. Moreover, mutations that inactivate individual members of this superfamily that are expressed in mammals result in a variety of inherited diseases. In this review, particular focus is given to the roles of Sec14-like proteins in coupling of phospholipid metabolism with specific membrane trafficking reactions, and with polarised membrane growth in various systems. In that regard, the yeast Sec14p is arguably the one member of the Sec14-superfamily for which there exists the largest body of functional information. The SEC14 structural gene was initially defined as a complementation group represented in the original collection of yeast secretory (sec) mutants [4]. Subsequent molecular characterization of the isolated gene demonstrated it is essential for the viability of yeast cells, and that the protein product is required for membrane trafficking from the yeast TGN [1]. In that regard, there is an interesting specificity to this requirement. Export of the secretory glycoprotein invertase is strongly compromised by Sec14p deficiency whereas trafficking of the vacuolar proteinase carboxypeptidase from the TGN into the endosomal system, and ultimately to the vacuole, is only mildly affected [1]. Thus, while Sec14p controls an essential trafficking pathway from the TGN, it is not required for all trafficking pathways from this Golgi subcompartment.
The Sec14p crystal structure
To fully ascertain how Sec14p binds to its phospholipid substrate, it would be most useful to determine crystal structures of Sec14p bound to either PtdIns or PtdCho. In that regard, that information is now forthcoming. The closest known Sec14p homologue (Sfh1p; see below) has been crystallized bound to PtdCho [6], and high resolution structures of Sfh1p bound to PtdEtn, PtdCho and PtdIns have now been solved (our unpublished data). The information from those structures will permit, for the first time, a comprehensive and rational structure/function analysis of Sec14p. The available Sec14p crystal structures represent ‘open’ Sec14p conformer where bound phospholipid has been exchanged with two molecules of the detergent βOG [7, 8]. Thus, the structure is considered to approximate that of an apo-Sec14p conformer that may transiently exist on a membrane surface once bound phospholipid has been unloaded but before another ligand molecule has infiltrated the hydrophobic pocket. The apo-Sec14p structure of Sec14p, while not particularly useful for discerning precise details regarding how Sec14p binds its substrates, nonetheless has been very useful in studies of conformational transitions that may be relevant to the reloading step in the Sec14p phosopholipid binding/exchange cycle (see below).
At the time its structure was solved, apo-Sec14p defined a novel fold comprised of twelve α-helices, six β-strands and eight 310-helices [Figure 1; 7]. These elements assemble into a compact fold that forms a hydrophobic pocket with a volume of 3000 Å3 which is sufficient in size to accommodate at most one phospholipid molecule. The hydrophobic pocket is stabilised by a region of the protein termed the “string motif”. This motif consists of both random coils and four 310 helices, extends around the back of the Sec14p molecule, and wraps around the β-strand floor of the lipid binding pocket. The Sec14p lipid binding domain is preceded by an N-terminal motif, consisting of α-helices 1-4, that associate to form the “tripod motif” that helps target the Sec14p molecule to Golgi membranes.
Figure 1. Crystal structure of an open Sec14p conformer.

The Sec14p fold is comprised of twelve α-helices, six β-strands and eight 310 helices. The hydrophobic pocket is formed by six β-strands and three α-helices (helices α8, α9 and α11; in grey) and this pocket is predicted to be gated by the A10/T4 helix (in blue). The lipid binding pocket of apo-Sec14p is occupied by 2 molecules of β-octylglucoside (in yellow) and these molecules are oriented such that the acyl chains project into the pocket, disposing the headgroup towards solvent. The N-terminal α1, α2, α3 and α4 helices form the “tripod motif” that helps target Sec14p to Golgi membranes (in green). The “string motif” (in red) is comprised of a random coil regions and four 310 helices. This structural element wraps around the back of the lipid binding domain and is critical both for protein stability and the conformational dynamics that accompany the phospholipid exchange cycle.
How is access to the hydrophobic pocket controlled? The lipid binding pocket is predicted to be gated by a structural module termed the A10/T4 helix, a hybrid α/310 surface helix. In this regard, the short acyl-chains of bound β-octylglucoside molecules form van der Waals contacts with A10/T4 [7], a point that will be revisited in the discussion of Sec14p conformational dynamics and the phospholipid-binding/exchange cycle (see below). Therefore, it is hypothesised that, as well as gating the binding pocket, the A10/T4 helix is functionally required during the phospholipid exchange reaction. Specifically, the hydrophobicity of the A10/T4 may promote its insertion into a membrane bilayer - thereby generating a condition favourable for ejection of bound phospholipid and reloading with another. Sophisticated EPR studies indeed suggest that the polarity gradient within the Sec14p lipid binding pocket provides a hydrophobic matching conducive for partitioning of a phospholipid molecule from the membrane bilayer into the binding pocket [9, 10]. How the candidate phospholipid ligand arrives to the point where this partitioning reaction is possible remains unresolved.
The apo-Sec14p crystal structure also suggests how phospholipid is oriented within the binding pocket. Bound phospholipid is predicted to be oriented such that the acyl chains are packed deep within the hydrophobic core of the pocket with the headgroup exposed to solvent [7, 8]. In support of this prediction, the PtdIns headgroup is accessible to the active site of PtdIns-3-kinase when bound to Sec14p [11]. This model has been further tested using EPR to investigate the dynamics of the PtdCho microenvironment within the Sec14p binding pocket [9, 10]. Bound PtdCho is highly immobilized within the Sec14p lipid binding pocket, and Sec14p binds the PtdCho headgroup/backbone region more tightly than the distal regions of the acyl chains. Moreover, solvent accessibility experiments indicate position C5-C12 of the bound PtdCho sn-2 acyl chain are shielded, while the distal region of the acyl chain shows small (but significant) accessibility to solvent [9, 10]. This is an enigmatic finding given the bulk of the evidence indicates the headgroup is solvent-exposed (see above). Extant possibilities include an unusual conformation of bound phospholipid within the Sec14p binding pocket, or that the distal sn-2 acyl chain also resides near the Sec14p surface.
Analysis of the Sec14p crystal structure suggests testable possibilities for how this protein interacts with the headgroup of PtdIns, at least. Scott Phillips, then a graduate student, posited the sugar headgroup of βOG molecule may serve as a reasonable model for the PtdIns inositol ring. The logic behind this hypothesis derived from the fact that the headgroup of one of the bound βOG molecules was coordinated via an elaborate hydrogen bonding network [8]. Using that headgroup as an inositol mimic, Phillips suggested E207 is a critical residue for coordination of the inositol ring. Proper positioning of E207 to fulfill such a role involved its electrostatic interaction with the side chain of K239. Finally, K66 was also posited to reside in the immediate vicinity of the inositol ring, and to facilitate its coordination within the Sec14p phospholipid binding pocket. These basic predictions were validated by structure/function analyses that report these residues to have an important and specific involvement for Sec14p PtdIns-transfer activity [8].
Conformational dynamics of Sec14p and phospholipid exchange
That helix A10/T4 may gate the phospholipid binding cavity of Sec14p was explicitly suggested when the apo-Sec14p structure was solved [7]. The essential correctness of this hypothesis was derived from structural studies of ‘closed’ conformers of other Sec14-like proteins [12, 13]. What remains unresolved is how the opening and closing of the gating helix is regulated. Exciting progress in that regard has recently come from a combination of MD simulations and functional analyses [14]. The simulations employed, as starting structure, a fully solvated ‘open’ Sec14p conformer derived from the apo-Sec14p crystal structure. It is thereby inferred that what is being modeled is the reloading stage of the Sec14p phospholipid exchange cycle.
The MD simulations model oscillating motions related to the closing and opening of the A10/T4/A11 helical gate. The conformational dynamics of the gate involve large rigid body motions that involve a hinge unit and a novel gating module (G-module) that transduces conformational information to the hinge [14]. Several important insights come from functional studies of the hinge unit. First, the hinge consists of elements dedicated to gate opening and gate closure. Second, closure of the gate appears to be a default pathway. Third, hinge residues involved in gate closure (e.g. K239) are also suggested to be intimately involved with phospholipid binding. Taken together, the MD simulation approach suggests apo-Sec14p engages a shallow ‘breathing’ regime described by rapidly oscillating partial closing and opening events that involve the helical gate. Upon encounter with a suitable phospholipid binding substrate, this breathing regime is induced to collapse down a conformational trajectory that incorporates phospholipid into the binding pocket and results in complete closure of the helical gate [14].
The G-module is an especially interesting element that consists of two distinct substructures B1LB2 (β-strand 1 : loop : β-strand 2) and A12LT5 (α-helix 12 : loop : 310 helix 5). The latter substructure is a component of the string motif described above that wraps around the back of the β-strand floor of the Sec14p hydrophobic pocket and the T5 helix is disrupted by the sec14-1ts missense mutation through which the SEC14 complementation group was first identified [7, 15]. How transduction of conformational information from the G-module to the hinge is regulated is an important and unresolved question as it speaks to the mechanism for how Sec14p opens the gate and exposes its phospholipid binding pocket. This is a reaction that must precede ejection of phospholipid from the binding pocket as an early step in the exchange cycle. The 114TDKDGR119 motif of B1LB2 is proposed to be central to the transduction process. Finally, the G-module is a site of a number of naturally occurring mutations that inactivate Sec14-like proteins or domains and result in mammalian disease [14]. This suggests the conformational dynamics pathways are hardwired similarly in all members of the Sec14-superfamily, and these disease-associated mutations result in defective exchange of bound lipophilic ligands.
Bypass Sec14p mutants and defects in PtdCho biosynthesis
We now appreciate that the essential role of Sec14p is to coordinate the critical interface between lipid metabolism and the activity of protein components that drive the biogenesis of secretory vesicles from the TGN. Precisely how Sec14p invests its phospholipid-binding/exchange activities remains to be elucidated. However, it is clear that Sec14p controls the lipid microenvironment of the TGN. A large body of evidence to that effect derives from the isolation of a remarkable class of loss-of-function mutations in nonessential genes, the ‘bypass Sec14’ mutations, that restore viability and secretory competence to yeast that are completely devoid of the normally essential Sec14p [3]. Bypass Sec14p mutations identify seven nonessential genes, and it is a logical principle that elucidation of how ‘bypass Sec14p’ mechanisms operate will prove directly informative regarding mechanisms for Sec14p function. As a visual guide to the concepts discussed below, a depiction of how ‘bypass Sec14p’ mutations fit into our present view of how Sec14p regulates TGN function is shown in Figure 2.
Figure 2. The Sec14p pathway.
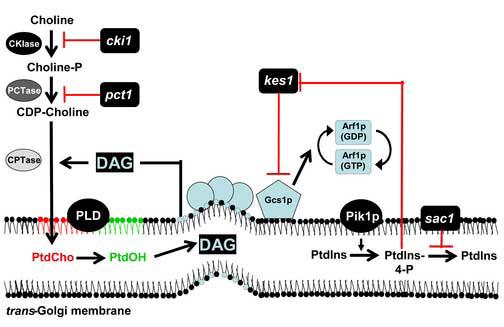
‘Bypass Sec14p’ mutants suppress sec14 defects. This schematic illustrates the various effects of ‘bypass Sec14p’ mutants on phospholipid metabolism. The identification of lipid metabolic/binding activities in the ‘bypass Sec14p’ collection demonstrate Sec14p regulates Golgi secretory function via control of lipid metabolism/membrane lipid content. One ultimate recipient of these regulatory effects is posited to be the adenosine diphosphate-ribosylation factor (ARF) cycle. Putative downstream targets of ‘bypass Sec14p’ mutants include the ARF-GAPs Gcs1p and Age2p. All ‘bypass Sec14p’ mutants require an active phospholipase D (PLD) to exert this distinguishing phenotype. Mutations in the structural genes for choline kinase (CKI1) and choline phosphate cytidylytransferase (PCT1) inhibit PtdCho production and reduce consumption of DAG into PtdCho biosynthesis. DAG and PtdCho are posited to represent ‘pro-secretory’ and ‘anti-secretory’ lipids, respectively, on the basis of their opposing effects on the activity of the pro-secretory Gcs1p/Age2p ARFGAP activities. Genetic disruption of the SAC1 gene, which encodes a phosphoinositide phosphatase, results in accumulation of PtdIns-4-P. PtdIns-4-P is categorized as a pro-secretory phospholipid and its synthesis in the Golgi complex is primarily catalyzed by the PtdIns 4-OK kinase Pik1p. Accessory proteins that modulate PtdIns-4-P signaling are not well characterized. One candidate is Kes1p, an oxysterol binding protein homolog. Genetic data identify Kes1p as either an inhibitor of PtdIns-4-P synthesis, an activator of PtdIns-4-P degradation, or a binding protein that limits PtdIns-4-P accessibility for pro-secretory signaling. Thus, Kes1p is a negative regulator of the Sec14p pathway. That PtdIns-4-P also regulates Kes1p function is also plausible.
An intriguing class of ‘bypass Sec14p’ mutations disrupts the activities of enzymes for PtdCho biosynthesis via the CDP-choline pathway [3] and, under conditions where choline salvage through the CDP-choline pathway is inhibited, structural genes for enzymes of the PtdEtn methylation pathway for PtdCho biosynthesis [16]. The significant involvement of PtdCho metabolism in the secretory function of Sec14p is further emphasized by the obligate requirement of the nonessential PLD enzyme for ‘bypass Sec14p’ [17, 18]. The relationship between Sec14p function and PtdCho metabolism is a satisfying one given PtdCho is a binding substrate for Sec14p. The genetic data are compatible with the general concept that Sec14p is required to detoxify the effects of PtdCho (or the active process of PtdCho synthesis via the CDP-choline pathway) on TGN-derived vesicle biogenesis [3]. Indeed, there is evidence suggesting that Sec14p directly alters PtdCho metabolism [8, 19, 20]. Loss of Sec14p function results in increased PtdCho levels in yeast TGN membranes [19].
Is PtdCho itself, or is the process of PtdCho biosynthesis, the culprit? Available evidence suggests both possibilities may be relevant. From the standpoint of PtdCho synthesis, the CDP-choline pathway is distinguished from the less ‘toxic’ PtdEtn methylation pathway by its stoichiometric consumption of DAG for every mole PtdCho synthesized [21]. By reducing metabolic flux through this pathway, Sec14p is poised to simultaneously effect reduction of PtdCho and increase DAG, and the PtdCho-bound form of Sec14p is proposed to contribute to such a regulatory function [8, 19, 20]. This basic concept is further supported by findings that exogenous short-chain DAG elicits a detectable pharmacological suppression of sec14-1ts secretory and growth defects [22, 23]. Collectively, these findings suggest a role for Sec14p in moderating activity of the CDP-choline pathway so that a favorable lipid environment for vesicular transport from the yeast TGN can be maintained [3, 8, 19, 20]. It is worth noting that inactivation of the biochemically analogous CDP-Etn pathway does not recapitulate the ‘bypass Sec14p’ phenotype associated with inactivation of the CDP-choline pathway. Why? This remains unclear. Possibilities include that flux through the CDP-choline pathway is more robust, that the ‘toxicity’ of the end product (PtdCho) to TGN exocytic function is different, or that the intracellular locations of those two biosynthetic pathways are sufficiently distinct (i.e one may be more highly represented in TGN membranes)..
Sac1p phosphoinositide phosphatase
How about the role of inositol phospholipids in Sec14p-mediated Golgi secretory function? Sec14p clearly facilitates phosphoinositide production in vivo [8, 24, 25]. Conspicuous in their absence in the collection of ‘bypass Sec14p’ mutants are mutations (or environmental conditions) that affect inositol or PtdIns synthesis. However, mutations in genes whose products regulate phosphoinositide homeostasis do exert ‘bypass Sec14p’. One class of ‘bypass Sec14p’ mutations inactivates Sac1p, an integral membrane protein of the Golgi and ER shown to regulate inositol phospholipid homeostasis [15, 22, 26]. Sac1p is a phosphoinositide phosphatase whose functional ablation results in elevation of PtdIns-3-P, PtdIns-3,5-P2, and particularly PtdIns-4-P levels in yeast [25, 27-30]. While it initially speculated that 10-fold elevation of bulk PtdIns-4-P in sac1 mutants is solely responsible for the ‘bypass Sec14p’ phenotype, the situation is more complicated and again highlights PtdCho metabolism. The elevated PtdIns-4-P levies no ‘bypass Sec14p’ effect in the absence of a catalytically active PLD (SPO14 gene product), and Sac1p defects also result in derangements of neutral lipid metabolism [25]. It seems more likely that the accumulation of PtdIns-4-P in sac1 mutants contributes to ‘bypass Sec14p’ by mislocalizing other phosphoinositide binding proteins that negatively impact the Sec14p pathway (i.e. Kes1p, see below; [31]). In support of this idea, Sac1p specifically degrades PtdIns-4-P generated by the non-Golgi PtdIns 4-OH kinase Stt4p rather than that PtdIns-4-P pool generated by the Golgi-associated PtdIns 4-OH Pik1p kinase [30].
An oxysterol binding protein and Sec14p-independent growth
An unanticipated link between Sec14p and sterol binding proteins arises from the demonstration that functional ablation of a yeast oxysterol binding protein homolog, Kes1p, results in ‘bypass Sec14p’ [32]. Kes1p is one of seven yeast members of the highly conserved oxysterol binding protein (OSBP) family recently shown to interact with CDC42 to promote cell polarity [33]. It is the sole yeast OSBP whose functional ablation results in ‘bypass Sec14p’ phenotypes [32, 34]. Given the Hes1p OSBP shares 70% sequence identity with Kes1p [34], it is surprising that Hes1p dysfunction does not evoke ‘bypass Sec14’ [32]. Reciprocally, a 2- to 4-fold increase in Kes1p dosage neutralizes ‘bypass Sec14p’ phenotypes associated with inactivation of the CDP-choline pathway for PtdCho biosynthesis [32]. Thus, the genetic data consistently identify Kes1p as a negative regulator of the Sec14p pathway.
Kes1p is a peripheral membrane protein of the yeast Golgi system. Targeting of Kes1p to the Golgi complex is sensitive to a phosphoinositide pool regulated by the Pik1p PtdIns-4OH kinase, and also requires a functional OSBP domain that is a highly conserved motif throughout the OSBP family [31, 36]. As mentioned above, mislocalization of Kes1p from Golgi membranes is recorded in sac1 mutants due to the massive accumulation of PtdIns-4-P in inappropriate compartments. Indeed, it is proposed that sac1-mediated ‘bypass Sec14p’ primarily reflects this mistargeting of Kes1p - an effect that phenocopies Kes1p loss-of-function [31]. While the biological function of Kes1p is not well characterized, the available evidence suggests Kes1p regulates the Pik1p PtdIns 4-OH kinase. Inactivation of Kes1p levies a specific genetic suppression of the growth defects associated with pik1ts mutations, but not stt4ts mutations [i.e. mutations in a yeast second PtdIns 4-OH kinase; 31]. These data are consistent with Kes1p either inhibiting the Pik1p PtdIns 4-OH kinase, activating a PtdIns-4-P phosphatase, clamping (sequestering) available PtdIns-4-P, or a combination of the above. The nature of Kes1p-mediated regulation of PtdIns-4-P ‘signaling’ requires further study, but genetic evidence suggests PtdIns-4-P may directly or indirectly modulate activity of the ARF small GTPase cycle in a manner that opposes how Sec14p regulates this cycle [31]. Another, and not mutually exclusive, possibility is Kes1p regulates a sterol component of TGN membranes, and that this component also contributes to the in vivo regulation of secretory vesicle biogenesis in a manner difficult to reproduce in vitro. Kes1p does not directly modulate the GTPase activating functions of ARF-GAPs (at least in vitro), nor does it exhibit any intrinsic ARF-GEF activity in vitro [37].
The various genetic and biochemical data highlight Sec14p-mediated regulation of both PtdCho and phosphoinositide metabolism as important biological properties for Sec14p function in yeast. While there have been differences of opinion regarding relative importance of these properties (e.g. whether Pik1p overproduction does or does not levy weak phenotypic suppression of sec14-1ts growth defects; 16,24), we suspect both arms of Sec14p function will ultimately prove important.
ARF-GAPs and Sec14p-dependent exocytosis
One candidate for a downstream effector of Sec14p-mediated regulation of lipid metabolism is Gcs1p, one of a pair of partially functionally redundant ARF-GAPs [31, 37]. This is at odds with general dogma that ARFGAPs are required only for the uncoating of transport vesicles in preparation for fusion with target membranes [38]. It is consistent with recent demonstrations that ARFGAPs contribute significantly to vesicle biogenesis. Mammalian reconstitution systems indicate a coupling of ARFGAPs with cargo sorting and packaging into nascent transport vesicles [39-42]. Furthermore, the yeast ARFGAP Glo3p, which shows partial functional redundancy with Gcs1p, is required for formation of vesicles that function in retrograde protein trafficking in vitro and in vivo [41].
A number of lines of evidence implicate the Gcs1p ARFGAP as acting downstream of Sec14p to promote Golgi secretion in Sec14p-dependent and Sec14p-independent pathways [37]. The lipid-sensitivity of Gcs1p ARFGAP activity is also consistent with this protein playing a role in the Sec14p pathway for TGN-derived secretory vesicle biogenesis. Gcs1p activity is negatively modulated by PtdCho and positively affected by DAG/PtdOH/PtdIns-4,5-P2 in vitro [37]. Of the activating lipids, present evidence favors DAG as a physiologically relevant player in the activation of Gcs1p in the yeast TGN. PtdIns-4,5-P2 binding is likely not essential in vivo given that Gcs1p lacking its PH-domain is completely functional in vivo [37], and high levels of PtdOH accumulation do not rescue sec14-1ts growth and secretory defects. How may DAG modulate Gcs1p ARFGAP activity? One possibility is that it contributes to the biophysical parameters that generate a suitable membrane microenvironment for vesicle budding. In that regard, DAG may induce a favorable membrane curvature instrumental for Gcs1p recruitment to membranes [43].
Non-classical yeast PITPs that regulate PtdIns metabolism
The Saccharomyces cerevisiae genome encodes for five genes whose protein products share at least 25% identity and 45% similarity to Sec14p, and these genes are termed Sec Fourteen Homologues 1-5 (SFH1-SFH5) [43]. Overexpression of SFH2, SFH4 or SFH5 phenotypically rescues the conditional lethality associated with the sec14-1ts mutation, and partially restores Golgi secretory function to the TGN of sec14-1ts cells [44,45]. These data indicate Sfh2p, Sfh4p and Sfh5p share some functional properties with Sec14p. In that regard, biochemical measurements record PtdIns-transfer activity for Sfh2p, Sfh3p, Sfh4p and Sfh5p, but PtdCho-transfer activity is low at best. The classification of these proteins as non-classical PITPs is based on their apparent inability to bind/transfer PtdCho in the face of measurable PtdIns-transfer activity.
The SFH proteins do not individually, nor collectively, execute essential cellular functions. Also, combinatorial deletion of the SFH genes in the sec14-1ts genetic background does not compromise cell viability -- suggesting that SFH proteins do not share functional redundancy with Sec14p [44]. However, SFH proteins as a group are required for Sec14p-independent cell growth in ‘bypass Sec14p’ mutants [44]. This phenotype is reminiscent of that associated with PLD deficiency as constitutive PLD activity is also required to support Sec14p independent cell growth in all ‘bypass Sec14p’ mutants [18]. This similarity is explained by demonstrations that optimal PLD activation in Sec14p-insufficient mutants requires the activities of SFH proteins [44]. Thus, while Sec14p deficiencies evoke PLD activation, deficiencies in SFH protein function result in failure to optimally activate PLD in vegetative cells. This situation appears to be reversed in sporulating cells [46], indicating the relationship between Sec14-like proteins and PLD signalling is subject to significant degrees of developmental regulation.
SFH proteins regulate phosphoinositide metabolism
Given that Sec14p expression stimulates phosphoinositide production in vivo, the biochemical properties of SFH proteins suggest a simple mechanism as to how these activate PLD. Namely, since PLD requires PtdIns-4,5-P2 for activity [47], that SFH proteins modulate the PtdIns-4,5-P2 pools that interface with PLD in vivo. Consistent with this idea, overexpression of SFH2 elevated both PtdIns-4-P and PtdIns-4,5-P2 in a yeast strain with basal phosphoinositide levels [48]. Overproduction of SFH4 and SFH5 had more modest effects that were limited to PtdIns-4,5-P2. Reciprocally, an sfhΔ mutant with combinatorial defects in Sfh protein activity exhibited a 40% reduction in bulk PtdIns-4, 5-P2 relative to isogenic wild-type controls [48]. Neither Sfh2p, Sfh4p nor Sfh5p directly stimulate the activity of yeast PtdIns 4-OH kinases (i.e. Pik1p, Stt4p and Lsb6p), nor do these stimulate the activity of Mss4p, the single yeast PtdIns-4-P 5-OH kinase [48]. Rather, genetic and biochemical analyses suggest SFH proteins (particularly Sfh2p and Sfh5p) stimulate phosphoinositide production by regulating delivery of PtdIns to the Stt4p PtdIns 4-OH kinase.
Organization of the yeast actin cytoskeleton is responsive both to phosphoinsoitide homeostasis and to the activity of the secretory pathway [49, 50], and Sec14p orthologues in Schizosaccharomyces pombe and Arabidopsis thaliana modulate the actin cytoskeleton as well [51, 52]. Both Sec14p and the SFH proteins contribute to actin organization. Sec14p dysfunction randomizes the normally polarized distribution of cortical actin patches in small budded cells and actin cables are diminished [48]. Overexpression of either SFH2 or SFH5 correct the actin derangements associated with Sec14p dysfunction. While en bloc functional ablation of SFH proteins does not levy a dramatic effect on bulk actin distribution in growing cells, it does reduce the efficiency at which the actin system reorganizes under conditions of osmotic stress [48]. Thus, SFH protein-mediated regulation of phosphoinositide homeostasis contributes to dynamic regulation of the actin cytoskeleton.
Sfh5p-mediated stimulation of PtdIns-4, 5-P2 synthesis and exocytosis
Stt4p and Mss4p localize to discrete plasma membrane domains [53], and functional evidence indicates SFH proteins collaborate with these PtdIns-kinases in optimizing the efficiency with which TGN-derived secretory vesicles target and dock to the plasma membrane. Overexpression of Sfh5p improves the efficiency with which exocytosis occurs in sec8-9ts, sec10-2ts and sec15-1ts late acting secretory mutants, and this effect is specific in that neither Sec14p not the other SFH proteins show this effect [48]. In this regard, overexpression of MSS4 or the t-SNARE Sec9p exerts similar effects, and the rescue evoked by increased dosage of these proteins requires a functional Sfh5p [48]. An attractive interpretation of these data is an Sfh5p/Stt4p/Mss4p-dependent plasma membrane pool of PtdIns-4,5-P2 regulates the activity of the Sec9p t-SNARE. Consistent with this view, Sfh5p is required for the plasma membrane targeting of a PLD-GFP chimera [48]. This chimera was previously shown to be a reliable reporter of elevated plasma membrane PtdIns-4,5-P2 when MSS4 gene dosage is increased [54].
SFH proteins and lipid trafficking via intermembrane contact sites
While Sfh5p functionally interacts with Stt4p, cell imaging experiments indicate that Sfh5p localizes to the peripheral endoplasmic reticulum and not the plasma membrane where Stt4p resides [48]. How does one account for these puzzling results? One possibility is SFH proteins regulate formation of intermembrane contact sites, thereby providing a nonvesicular mechanism of regulating lipid transfer between distinct intracellular membrane systems. The phospholipid binding activity of Sfh5p may ‘gate’ the contact site by presenting passenger phospholipids to the site, or Sfh5p may play a more direct role in the formation of the contact site (Figure 3).
Figure 3. An intermembrane contact site model for SFH protein function.

(A) SFH proteins (grey) may themselves be integral components of intermembrane contact sites, employing their intrinsic PtdIns-binding capacity (in black) to traffic PtdIns through a portal. (B) An SFH protein may employ its ability to present PtdIns to a phosphoinositide kinase to generate a phosphoinositide platform that recruits intrinsic component(s) (Factor X) of an intermembrane contact site. In this scenario, the SFH protein does not directly utilize its PtdIns-binding properties to impose PtdIns-trafficking specificity through such a site. Other lipid species could pass through such a site. This model most likely applies to the case of Sfh4p-dependent trafficking of PtdSer to an extramitochondrial PtdSer decarboxylase [55; see text].
This idea may generally apply to SFH proteins as these proteins are rather tightly membrane-bound [44, 55]. Moreover, Sfh4p (also termed PstB2p/Pdr17p) is implicated in nonvesicular transport of PtdSer from the ER to an extramitochondrial PtdSer decarboxylase that converts PtdSer to PtdEtn [55]. Potentiation of this intriguing metabolic pathway is specific to Sfh4p. Neither Sec14p, Sfh2p, Sfh3p nor Sfh5p substitute for Sfh4p in this metabolic pathway [48]. That SFH proteins represent specialized components of intermembrane contact sites is an attractive one.
Higher Plant Sec14 proteins
As introduced above, Sec14-like proteins are ubiquitously and generously distributed across the entire breadth of the eukaryotic kingdom. Many of these Sec14-like proteins have additional interesting domains, such as nodulin and GOLD domains, appended to them. These more complex Sec14-like proteins are generally found only in multicellular eukaryotes and these are highly represented in plant systems. In that regard, the genetic tractability of Arabidopsis thaliana provides a unique opportunity to investigate the functions of multidomain Sec14-like proteins. The genome of A. thaliana encodes for 31 distinct open reading frames whose products display significant homology to Sec14p (AtSFH proteins; for an alignment see [4, 52]). At least twelve consist of a Sec14p domain positioned upstream of a nodulin domain, whereas the remainder consist of either a Sec14-domain alone or a Sec14-domain followed by a GOLD domain (Figure 4). We will focus on the Sec14-nodulin and Sec14-GOLD proteins.
Figure 4. Sec14p like proteins in Arabidopsis thaliana.

Bioinformatic analysis of the A. thaliana genome has identified at least 31 ORFs to encode proteins that share significant homology to Sec14p. Twelve ORFs consist of a Sec14p like domain positioned upstream of a nodulin domain whereas the remaining nineteen Sec14p like proteins consist of either a Sec14p lipid binding domain (LBD) alone or a Sec14-like LBD that precedes a GOLD domain.
Novel Sec14-nodulin PITPs in Arabidopsis thaliana
Recent evidence suggests AtSFH proteins, particularly the Sec14-nodulin two-domain proteins, may be generally required for developmentally regulated pathways for membrane morphogenesis. A striking demonstration to this effect comes from a comprehensive analysis of one of these Arabidopsis proteins, AtSfh1p, and of its homologs in the leguminous plant Lotus japonicus [52, 56]. Root hair development in A. thaliana requires highly regulated polarized membrane growth from a precise position on the epidermal plasma membrane of the root trichoblast cells. This developmental morphogenetic pathway is highlighted by a tightly polarized trafficking of membrane to the growing root tip, thereby ensuring proper root hair elongation. The Sec14-nodulin protein AtSfh1p is required for this process as functional ablation of this protein leads to disorganized membrane deposition during root hair growth and results in short deformed root hairs [Figure 5; 52]. The modular design of AtSfh1p reports domain-specific functions. The Sec14-domain has intrinsic PtdIns- and PtdCho-transfer and stimulates synthesis of 4-OH phosphoinositides, i.e. PtdIns-4-P and PtdIns-4,5-P2, in Sec14p-deficient yeast [51]. The nodulin domain is a membrane targeting unit and both domains are required for a functional AtSfh1p [52, 56].
Figure 5. AtSfh1p is required for root hair development in A. thaliana.

Light microscopy of living, 10 day old, wild-type (left) and nullizygous Atsfh1-/- seedlings (right). The defects in polarized membrane trafficking to the growing root tip in Atsfh1-/- plants manifest themselves in the obvious short root hair phenotype of Atsfh1-/- seedlings. Bars, 430μm.
How does AtSfh1p execute biological function? High-resolution vital imaging experiments indicate the localization of AtSfh1p correlates with the highly non-random distribution of PtdIns-4,5-P2 on the tip plasma membrane and on what are thought to be secretory vesicles [52]. This PtdIns-4,5-P2 organization is compromised in Atsfh1 nullizygous plants and, together with this defect, striking derangements of the normally tip-directed Ca++ gradient, the tip actin cytoskeleton, and the root hair microtubule cytoskeleton are also recorded. These various defects are hypothesized to arise primarily from collapse of a PtdIns-4,5-P2-dependent polarized membrane trafficking pathway [52]. That other Sec14-nodulin proteins are most highly expressed in tissues exhibiting highly polarized membrane growth (e.g. pollen tubes) suggests the principles gleaned from AtSfh1p will apply generally across this unusual family of more complex Sec14-like proteins.
The nodulin domains are interesting modules in their own right. In particular, their extreme C-termini are clamped by stretches of basic residues [57]. These are reminiscent of the polybasic motifs involved in phosphoinositide binding [58], and raise the intriguing possibility that Sec14-nodulin proteins not only have the ability to stimulate phosphoinositide synthesis but are also able to organize phosphoinositides by sequestering them in high-affinity nodulin domain-phospholipid interactions. This potential design suggests biologically useful mechanisms for building a membrane nanocircuitry suitable for very fine spatial and temporal regulation of phosphoinositide signaling [57].
Sec14-GOLD proteins
The GOLD domain was first characterized on the basis of its homology to the lumenal domain of KE8E4.6 in Caenorhabditis elegans, a member of the p24 protein family [59]. The p24 proteins are cargo receptors that select and concentrate proteins into nascent transport vesicles [60]. In all known instances of Sec14-GOLD proteins, the Sec14-domain precedes the GOLD domain. Given the role of Sec14-like proteins (and PITPs) in membrane trafficking, the linkage of Sec14-domains with cargo receptor-related GOLD-domains is intriguing to say the least. Arabidopsis expresses six Sec14-GOLD proteins while mammals express five. In all cases, the Sec14-domains of Sec14-GOLD proteins are more distantly related to yeast Sec14p than Sec14-nodulin proteins for instance [4]. Nonetheless, Sec14p residues essential for PtdIns binding (i.e. E207, K239) are often conserved. For example, these residues are conserved in all six Arabidopsis Sec14-GOLD proteins (patellins; PATL1-PATL6). The significance of this remains to be determined, but PATL1 reportedly binds to phosphoinositides in preference to either PtdEtn or PtdCho [61]. It is not yet unknown whether the Sec14-domain or the GOLD-domain is the phosphoinositide binding module. Based on intracellular localization data, PATL1 is suggested to play a role in cytokinesis; a process where actin and PtdIns-4,5-P2 are certainly involved. Whether PATL1 is genuinely involved in cytokinesis awaits genetic studies with nullizygous plants.
Plant Sec14p and the hyperosmotic stress response
Two Sec14-like proteins sharing 25% identity with yeast Sec14p have been identified in Oryza sativa [62]. These proteins, Ssh1p and Ssh2p, bind to phosphoinositides, particularly PtdIns-3,5-P2, with reasonable affinity. PtdIns-3,5-P2 synthesis is strongly stimulated by hyperosmotic stress in eukaryotes [63]. In that regard, Ssh1p is rapidly phosphorylated specifically under conditions of hyperosmotic stress when expressed in plants or even in S. cerevisiae [62]. The responsible kinases are the hyperosmotic stress response kinases SPK1 and SPK2, and Ssh1p phosphorylation is limited to those cells in a tissue that are challenged with the stress [64]. This phosphorylation is uncharacterized, but it does release Ssh1p from membranes to the cytosol [62,64]. Together, these findings are consistent with the concept that Oryza sativa Ssh1p regulates signalling in response to hyperosmotic stress. Whether phosphorylation regulates the ability of Ssh1p to bind to phosphoinositides, or whether this modification releases Ssh1p from membranes to promote other signalling reactions remains to be determined. These findings do suggest the possibility that other Sec14-like proteins also regulate the interface between lipid metabolism and response of various stress challenges.
Concluding Remarks
Sec14-like PITPs are a highly conserved and ubiquitous class of eukaryotic proteins that regulate the interface between lipid metabolism and cellular function. The recent progress in important aspects of the mechanistic ‘enzymology’ of Sec14p emphasizes the powerful contributions structural and modeling approaches will continue to bring to the problem. A number of questions still remain. These include the question of how is activity of the G-module regulated so the conformational transitions required for the phospholipid binding/exchange cycle are appropriately regulated? Is the conformational trajectory involved in ejection of bound phospholipid perfectly symmetrical with that which accompanies the reloading subreaction of the exchange cycle? The biology of Sec14-like PITPs will certainly prove to be an interesting subject. It is likely the diversity of the ligand binding capacities of these proteins is not fully appreciated. The mechanisms through which ligand binding/exchange is linked to physiological function are also likely to be varied. Finally, while the involvement of Sec14-like PITPs in membrane trafficking and cytoskeleton dynamics may prove a common functional theme, the physiological functions of Sec14-like protein functions are also likely to prove highly diverse. It is with keen anticipation that we await the new discoveries that are no doubt forthcoming in this arena.
Abbreviations
- ARF
ADP ribosylation factor
- ARF-GAPs
ARF-GTPase-activating proteins
- βOG
β-octylglucoside
- CDP
cytidine-diphosphate
- DAG
diacylglycerol
- EPR
electron paramagnetic resonance
- ER
endoplasmic reticulum
- Etn
ethanolamine
- GFP
green fluorescent protein
- MD
molecular dynamics
- OSBP
oxysterol binding protein
- PtdOH
phosphatidic acid
- PtdIns
phosphatidylinositol
- PITP
PtdIns/PtdCho-transfer protein
- PtdCho
phosphatidylcholine
- PLD
phospholipase D
- PtdEtn
phosphatidylethanolamine
- PtdSer
phosphatidylserine
- SFH
Sec Fourteen Homologue
- TGN
trans-Golgi Network
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bankaitis VA, Malehorn DE, Emr SD, Greene R. The Saccharomyces cerevisiae SEC14 gene encodes a cytosolic factor that is required for transport of secretory proteins from the yeast Golgi complex. J Cell Biol. 1989;108(4):1271–81. doi: 10.1083/jcb.108.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bankaitis VA, Aitken JR, Cleves AE, Dowhan W. An essential role for a phospholipid transfer protein in yeast Golgi function. Nature. 1990;347(6293):561–2. doi: 10.1038/347561a0. [DOI] [PubMed] [Google Scholar]
- [3].Cleves AE, McGee TP, Whitters EA, Champion KM, Aitken JR, Dowhan W, Goebl M, Bankaitis VA. Mutations in the CDP-choline pathway for phospholipid biosynthesis bypass the requirement for an essential phospholipid transfer protein. Cell. 1991;64(4):789–800. doi: 10.1016/0092-8674(91)90508-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Phillips SE, Vincent P, Rizzieri KE, Schaaf G, Gaucher EA, Bankaitis VA. The diverse biological functions of phosphatidylinositol transfer proteins in eukaryotes. Crit Rev Biochem Mol Biol. 2005;41(1):21–49. doi: 10.1080/10409230500519573. [DOI] [PubMed] [Google Scholar]
- [5].Novick P, Field C, Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21(1):205–15. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- [6].Schaaf G, Betts L, Garrett TA, Raetz CR, Bankaitis VA. Crystallization and preliminary X-ray diffraction analysis of phospholipid-bound Sfh1p, a member of the Saccharomyces cerevisiae Sec14p-like phosphatidylinositol transfer protein family. Acta Crystallograph Sect F Struct Biol Cryst Commun. 2006;62(11):1156–60. doi: 10.1107/S1744309106041728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sha B, Phillips SE, Bankaitis VA, Luo M. Crystal structure of the Saccharomyces cerevisiae phosphatidylinositol-transfer protein. Nature. 1998;391(6666):506–10. doi: 10.1038/35179. [DOI] [PubMed] [Google Scholar]
- [8].Phillips SE, Sha B, Topalof L, Xie Z, Alb JG, Klenchin VA, Swigart P, Cockcroft S, Martin TF, Luo M, Bankaitis VA. Yeast Sec14p deficient in phosphatidylinositol transfer activity is functional in vivo. Mol Cell. 1999;4(2):187–97. doi: 10.1016/s1097-2765(00)80366-4. [DOI] [PubMed] [Google Scholar]
- [9].Smirnova TI, Chadwick TG, MacArthur R, Poluektov O, Song L, Ryan MM, Schaaf G, Bankaitis VA. The chemistry of phospholipid binding by the Saccharomyces cerevisiae phosphatidylinositol transfer protein Sec14p as determined by EPR spectroscopy. J Biol Chem. 2006;281(46):34897–908. doi: 10.1074/jbc.M603054200. [DOI] [PubMed] [Google Scholar]
- [10].Smirnova TI, Chadwick TG, van Tol J, Ozarowski A, Poluektov O, Schaaf G, Ryan MM, Bankaitis VA. Local polarity and hydrogen bonding inside the Sec14p phospholipid-binding cavity: High-field multifrequency studies. Biophys. J. 2007 doi: 10.1529/biophysj.106.097899. (In Press).
- [11].Jones SM, Alb JG, Jr, Phillips SE, Bankaitis VA, Howell KE. A phosphatidylinositol 3-kinase and phosphatidylinositol transfer protein act synergistically in formation of constitutive transport vesicles from the trans-Golgi network. J Biol Chem. 1998;273(17):10349–54. doi: 10.1074/jbc.273.17.10349. [DOI] [PubMed] [Google Scholar]
- [12].Stocker A, Tomizaki T, Schulze-Briese C, Baumann U. Crystal structure of the human supernatant protein factor. Structure. 2002;10:1533–1540. doi: 10.1016/s0969-2126(02)00884-5. [DOI] [PubMed] [Google Scholar]
- [13].Min KC, Kovall RA, Hendrickson WA. Crystal structure of α-tocopherol transfer protein bound to its ligand: Implications for ataxia with vitamin E deficiency. Proc. Natl. Acad. Sci. 2003;100:14713–14718. doi: 10.1073/pnas.2136684100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ryan MM, Temple BRS, Phillips SE, Bankaitis VA. Conformational dynamics of the major yeast phosphatidylinositol transfer protein Sec14p: Insights into the mechanisms of phospholipid exchange and diseases of Sec14p-like protein deficiencies. Mol. Biol. Cell. 2007 doi: 10.1091/mbc.E06-11-1024. (In Press, published March 7, 2007 as 10.1091/mbc.E06-11-1024).
- [15].Cleves AE, Novick PJ, Bankaitis VA. Mutations in the SAC1 gene suppress defects in yeast Golgi and yeast actin function. J Cell Biol. 1989;109(6 Pt 1):2939–50. doi: 10.1083/jcb.109.6.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Xie Z, Fang M, Bankaitis VA. Evidence for an intrinsic toxicity of phosphatidylcholine to Sec14p-dependent protein transport from the yeast Golgi complex. Mol Biol Cell. 2001;12(4):1117–29. doi: 10.1091/mbc.12.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sreenivas A, Patton-Vogt JL, Bruno V, Griac P, Henry SA. A role for phospholipase D (Pld1p) in growth, secretion, and regulation of membrane lipid synthesis in yeast. J Biol Chem. 1998;273(27):16635–8. doi: 10.1074/jbc.273.27.16635. [DOI] [PubMed] [Google Scholar]
- [18].Xie Z, Fang M, Rivas MP, Faulkner AJ, Sternweis PC, Engebrecht JA, Bankaitis VA. Phospholipase D activity is required for suppression of yeast phosphatidylinositol transfer protein defects. Proc Natl Acad Sci U S A. 1998;95(21):12346–51. doi: 10.1073/pnas.95.21.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].McGee TP, Skinner HB, Whitters EA, Henry SA, Bankaitis VA. A phosphatidylinositol transfer protein controls the phosphatidylcholine content of yeast Golgi membranes. J Cell Biol. 1994;124(3):273–87. doi: 10.1083/jcb.124.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Skinner HB, McGee TP, McMaster CR, Fry MR, Bell RM, Bankaitis VA. The Saccharomyces cerevisiae phosphatidylinositol-transfer protein effects a ligand-dependent inhibition of choline-phosphate cytidylyltransferase activity. Proc Natl Acad Sci U S A. 1995;92(1):112–6. doi: 10.1073/pnas.92.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Carman GM, Zeimetz GM. Regulation of phospholipid biosynthesis in the yeast Saccharomyces cerevisiae. J Biol Chem. 1996;271(23):13293–6. doi: 10.1074/jbc.271.23.13293. [DOI] [PubMed] [Google Scholar]
- [22].Kearns BG, McGee TP, Mayinger P, Gedvilaite A, Phillips SE, Kagiwada S, Bankaitis VA. Essential role for diacylglycerol in protein transport from the yeast Golgi complex. Nature. 1997;387(6628):101–5. doi: 10.1038/387101a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Henneberry AL, Lagace TA, Ridgway ND, McMaster CR. Phosphatidylcholine synthesis influences the diacylglycerol homeostasis required for Sec14p-dependent Golgi function and cell growth. Mol. Biol. Cell. 2001;12:511–20. doi: 10.1091/mbc.12.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hama H, Schnieders EA, Thorner J, Takemoto JY, DeWald DB. Direct involvement of phosphatidylinositol 4-phosphate in secretion in the yeast Saccharomyces cerevisiae. J Biol Chem. 1999;274(48):34294–300. doi: 10.1074/jbc.274.48.34294. [DOI] [PubMed] [Google Scholar]
- [25].Rivas MP, Kearns BG, Xie Z, Guo S, Sekar MC, Hosaka K, Kagiwada S, York JD, Bankaitis VA. Pleiotropic alterations in lipid metabolism in yeast sac1 mutants: relationship to “bypass Sec14p” and inositol auxotrophy. Mol Biol Cell. 1999;10(7):2235–50. doi: 10.1091/mbc.10.7.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Whitters EA, Cleves AE, McGee TP, Skinner HB, Bankaitis VA. SAC1p is an integral membrane protein that influences the cellular requirement for phospholipid transfer protein function and inositol in yeast. J Cell Biol. 1993;122(1):79–94. doi: 10.1083/jcb.122.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Guo S, Stolz LE, Lemrow SM, York JD. SAC1-like domains of yeast SAC1, INP52, and INP53 and of human synaptojanin encode polyphosphoinositide phosphatases. J Biol Chem. 1999;274(19):12990–5. doi: 10.1074/jbc.274.19.12990. [DOI] [PubMed] [Google Scholar]
- [28].Stock SD, Hama H, DeWald DB, Takemoto JY. SEC14-dependent secretion in Saccharomyces cerevisiae. Nondependence on sphingolipid synthesis-coupled diacylglycerol production. J Biol Chem. 1999;274(19):12979–83. doi: 10.1074/jbc.274.19.12979. [DOI] [PubMed] [Google Scholar]
- [29].Hughes WE, Cooke FT, Parker PJ. Sac phosphatase domain proteins. Biochem J. 2000;350(2):337–52. [PMC free article] [PubMed] [Google Scholar]
- [30].Nemoto Y, Kearns BG, Wenk MR, Chen H, Mori K, Alb JG, Jr, De Camilli P, Bankaitis VA. Functional characterization of a mammalian Sac1 and mutants exhibiting substrate-specific defects in phosphoinositide phosphatase activity. J Biol Chem. 2000;275(44):34293–305. doi: 10.1074/jbc.M003923200. [DOI] [PubMed] [Google Scholar]
- [31].Li X, Rivas MP, Fang M, Marchena J, Mehrotra B, Chaudhary A, Feng L, Prestwich GD, Bankaitis VA. Analysis of oxysterol binding protein homologue Kes1p function in regulation of Sec14p-dependent protein transport from the yeast Golgi complex. J Cell Biol. 2002;157(1):63–77. doi: 10.1083/jcb.200201037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fang M, Kearns BG, Gedvilaite A, Kagiwada S, Kearns M, Fung MK, Bankaitis VA. Kes1p shares homology with human oxysterol binding protein and participates in a novel regulatory pathway for yeast Golgi-derived transport vesicle biogenesis. EMBO J. 1996;15(23):6447–59. [PMC free article] [PubMed] [Google Scholar]
- [33].Kozminski KG, Alfaro G, Dighe S, Beh CT. Homologues of oxysterol-binding proteins affect Cdc42p- and Rho1p-mediated cell polarization in Saccharomyces cerevisiae. Traffic. 2006;7(9):1224–42. doi: 10.1111/j.1600-0854.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- [34].Beh CT, Cool L, Phillips J, Rine J. Overlapping functions of the yeast oxysterol-binding protein homologues. Genetics. 2001;157(3):1117–40. doi: 10.1093/genetics/157.3.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Jiang B, Brown JL, Sheraton J, Fortin N, Bussey H. A new family of yeast genes implicated in ergosterol synthesis is related to the human oxysterol binding protein. Yeast. 1994;10(3):341–53. doi: 10.1002/yea.320100307. [DOI] [PubMed] [Google Scholar]
- [36].Im YJ, Raychaudhuri S, Prinz WA, Hurley JH. Structural mechanism for sterol sensing and transport by OSBP-related proteins. Nature. 2005;437(7055):154–8. doi: 10.1038/nature03923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yanagisawa LL, Marchena J, Xie Z, Li X, Poon PP, Singer RA, Johnston GC, Randazzo PA, Bankaitis VA. Activity of specific lipid-regulated ADP ribosylation factor-GTPase-activating proteins is required for Sec14p-dependent Golgi secretory function in yeast. Mol Biol Cell. 2002;13(7):2193–206. doi: 10.1091/mbc.01-11-0563.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tanigawa G, Orci L, Amherdt M, Ravazzola M, Helms JB, Rothman JE. Hydrolysis of bound GTP by ARF protein triggers uncoating of Golgi-derived COP-coated vesicles. J Cell Biol. 1993;123(6 Pt 1):1365–71. doi: 10.1083/jcb.123.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rein U, Andag U, Duden R, Schmitt HD, Spang A. ARF-GAP-mediated interaction between the ER-Golgi v-SNAREs and the COPI coat. J Cell Biol. 2002;157(3):395–404. doi: 10.1083/jcb.200112092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yang JS, Lee SY, Gao M, Bourgoin S, Randazzo PA, Premont RT, Hsu VW. ARFGAP1 promotes the formation of COPI vesicles, suggesting function as a component of the coat. J Cell Biol. 2002;159(1):69–78. doi: 10.1083/jcb.200206015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lewis SM, Poon PP, Singer RA, Johnston GC, Spang A. The ArfGAP Glo3 is required for the generation of COPI vesicles. Mol Biol Cell. 2004;15(9):4064–72. doi: 10.1091/mbc.E04-04-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lee SY, Yang JS, Hong W, Premont RT, Hsu VW. ARFGAP1 plays a central role in coupling COPI cargo sorting with vesicle formation. J Cell Biol. 2005;168(2):281–90. doi: 10.1083/jcb.200404008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Bigay J, Casella JF, Drin G, Mesmin B, Antonny B. ArfGAP1 responds to membrane curvature through the folding of a lipid packing sensor motif. EMBO J. 2005;24(13):2244–53. doi: 10.1038/sj.emboj.7600714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Li X, Routt SM, Xie Z, Cui X, Fang M, Kearns MA, Bard M, Kirsch DR, Bankaitis VA. Identification of a novel family of nonclassic yeast phosphatidylinositol transfer proteins whose function modulates phospholipase D activity and Sec14p-independent cell growth. Mol Biol Cell. 2000;11(6):1989–2005. doi: 10.1091/mbc.11.6.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Schnabl M, Oskolkova OV, Holic R, Brezna B, Pichler H, Zagorsek M, Kohlwein SD, Paltauf F, Daum G, Griac P. Subcellular localization of yeast Sec14 homologues and their involvement in regulation of phospholipid turnover. Eur J Biochem. 2003;270(15):3133–45. doi: 10.1046/j.1432-1033.2003.03688.x. [DOI] [PubMed] [Google Scholar]
- [46].Rudge SA, Zhou C, Engebrecht J. Differential regulation of Saccharomyces cerevisiae phospholipase D in sporulation and Sec14-independent secretion. Genetics. 2002;160(4):1353–61. doi: 10.1093/genetics/160.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Sciorra VA, Rudge SA, Wang J, McLaughlin S, Engebrecht J, Morris AJ. Dual role for phosphoinositides in regulation of yeast and mammalian phospholipase D enzymes. J Cell Biol. 2002;159(6):1039–49. doi: 10.1083/jcb.200205056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Routt SM, Ryan MM, Tyeryar K, Rizzieri KE, Mousley C, Roumanie O, Brennwald PJ, Bankaitis VA. Nonclassical PITPs activate PLD via the Stt4p PtdIns-4-kinase and modulate function of late stages of exocytosis in vegetative yeast. Traffic. 2005;6(12):1157–72. doi: 10.1111/j.1600-0854.2005.00350.x. [DOI] [PubMed] [Google Scholar]
- [49].Goldschmidt-Clermont PJ, Machesky LM, Baldassare JJ, Pollard TD. The actin-binding protein profilin binds to PIP2 and inhibits its hydrolysis by phospholipase C. Science. 1990;247(4950):1575–8. doi: 10.1126/science.2157283. [DOI] [PubMed] [Google Scholar]
- [50].Roumanie O, Wu H, Molk JN, Rossi G, Bloom K, Brennwald P. Rho GTPase regulation of exocytosis in yeast is independent of GTP hydrolysis and polarization of the exocyst complex. J Cell Biol. 2005;170(4):583–94. doi: 10.1083/jcb.200504108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Nakase Y, Nakamura T, Hirata A, Routt SM, Skinner HB, Bankaitis VA, Shimoda C. The Schizosaccharomyces pombe spo20(+) gene encoding a homologue of Saccharomyces cerevisiae Sec14 plays an important role in forespore membrane formation. Mol Biol Cell. 2001;(4):901–17. doi: 10.1091/mbc.12.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Vincent P, Chua M, Nogue F, Fairbrother A, Mekeel H, Xu Y, Allen N, Bibikova TN, Gilroy S, Bankaitis VA. A Sec14p-nodulin domain phosphatidylinositol transfer protein polarizes membrane growth of Arabidopsis thaliana root hairs. J Cell Biol. 2005;168(5):801–12. doi: 10.1083/jcb.200412074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Audhya A, Emr SD. Stt4 PI 4-kinase localizes to the plasma membrane and functions in the Pkc1-mediated MAP kinase cascade. Dev. Cell. 2002;2(5):593–605. doi: 10.1016/s1534-5807(02)00168-5. [DOI] [PubMed] [Google Scholar]
- [54].Coluccio A, Malzone M, Neiman AM. Genetic evidence of a role for membrane lipid composition in the regulation of soluble NEM-sensitive factor receptor function in Saccharomyces cerevisiae. Genetics. 2004;166(1):89–97. doi: 10.1534/genetics.166.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wu WI, Routt S, Bankaitis VA, Voelker DR. A new gene involved in the transport-dependent metabolism of phosphatidylserine, PSTB2/PDR17, shares sequence similarity with the gene encoding the phosphatidylinositol/phosphatidylcholine transfer protein, SEC14. J Biol Chem. 2000;275(19):14446–56. doi: 10.1074/jbc.275.19.14446. [DOI] [PubMed] [Google Scholar]
- [56].Kapranov P, Routt SM, Bankaitis VA, de Bruijn FJ, Szczyglowski K. Nodule-specific regulation of phosphatidylinositol transfer protein expression in Lotus japonicus. Plant Cell. 2001;(6):1369–82. doi: 10.1105/tpc.13.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ile KE, Schaaf G, Bankaitis VA. Phosphatidylinositol transfer proteins and cellular nanoreactors for lipid signaling. Nat Chem Biol. 2006;2(11):576–83. doi: 10.1038/nchembio835. [DOI] [PubMed] [Google Scholar]
- [58].McLaughlin S, Murray D. Plasma membrane phosphoinositide organization by protein electrostatics. Nature. 2005;438(7068):605–11. doi: 10.1038/nature04398. [DOI] [PubMed] [Google Scholar]
- [59].Anantharaman V, Aravind L. The GOLD domain, a novel protein module involved in Golgi function and secretion. Genome Biol. 2002;3(5):0023. doi: 10.1186/gb-2002-3-5-research0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Springer S, Chen E, Duden R, Marzioch M, Rowley A, Hamamoto S, Merchant S, Schekman R. The p24 proteins are not essential for vesicular transport in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 2000;97(8):4034–9. doi: 10.1073/pnas.070044097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Peterman TK, Ohol YM, McReynolds LJ, Luna EJ. Patellin1, a novel Sec14-like protein, localizes to the cell plate and binds phosphoinositides. Plant Physiol. 2004;136(2):3080–94. doi: 10.1104/pp.104.045369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Kearns MA, Monks DE, Fang M, Rivas MP, Courtney PD, Chen J, Prestwich GD, Theibert AB, Dewey RE, Bankaitis VA. Novel developmentally regulated phosphoinositide binding proteins from soybean whose expression bypasses the requirement for an essential phosphatidylinositol transfer protein in yeast. EMBO J. 1998;17(14):4004–17. doi: 10.1093/emboj/17.14.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Dove SK, Cooke FT, Douglas MR, Sayers LG, Parker PJ, Michell RH. Osmotic stress activates phosphatidylinositol-3,5-bisphosphate synthesis. Nature. 1997;390(6656):187–92. doi: 10.1038/36613. [DOI] [PubMed] [Google Scholar]
- [64].Monks DE, Aghoram K, Courtney PD, DeWald DB, Dewey RE. Hyperosmotic stress induces the rapid phosphorylation of a soybean phosphatidylinositol transfer protein homolog through activation of the protein kinases SPK1 and SPK2. Plant Cell. 2001;13(5):1205–19. doi: 10.1105/tpc.13.5.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]


