Abstract
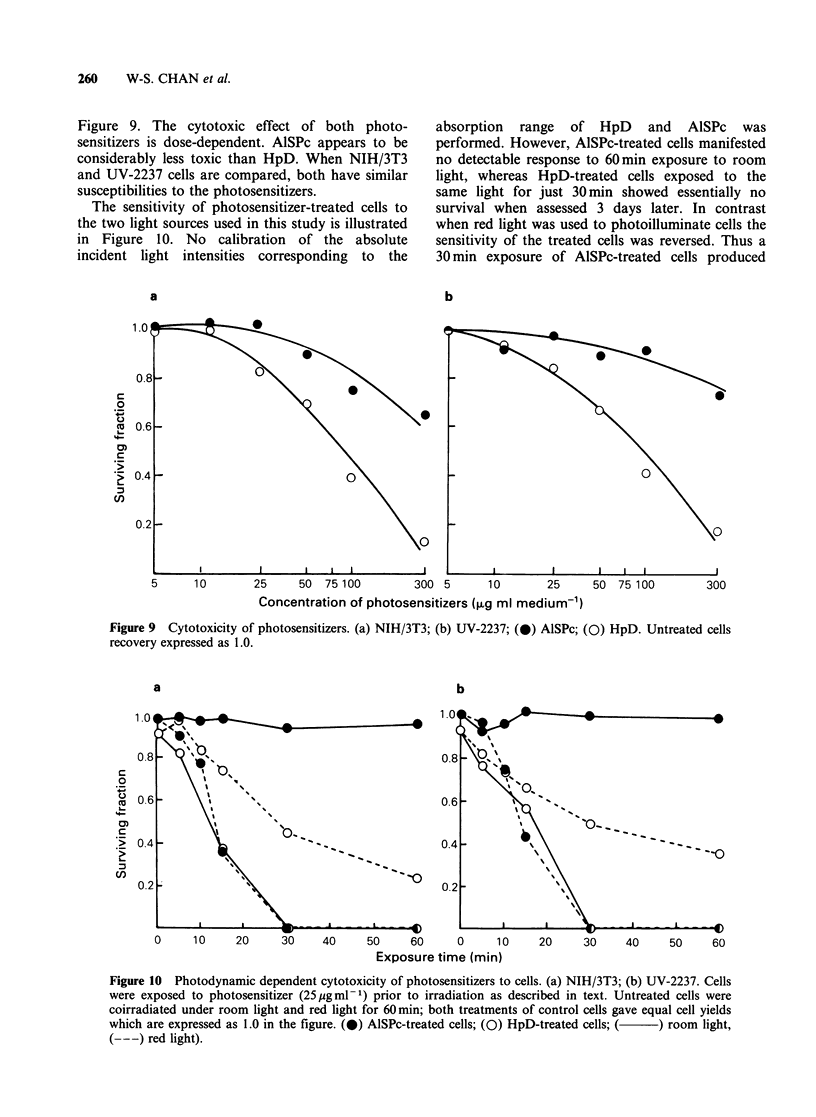
The uptake, retention and effects of aluminium chloro sulphonated phthalocyanine (AlSPc) were measured in two cell lines, UV-2237 a murine fibrosarcoma and the non-tumorigenic NIH/3T3 fibroblast line. The behaviour of cells treated with AlSPc was compared with that of those treated with haematoporphyrin derivative (HpD), a photosensitizer often used in photodynamic therapy (PDT) of cancer. AlSPc absorbs light strongly in the red region, is taken up by cells in a dose dependent fashion and is retained in vitro over a period of days (5 days after exposure greater than 40% remains cell-associated versus less than 25% of HpD). Additionally AlSPc was less cytotoxic to cells, maintained in darkness or exposed to room light, compared to HpD (100% viability versus 0% viability 3 days after 60 min exposure to room light). However red light (approximately 600-700 nm) caused greater toxicity in AlSPc-treated cells (100%) than in similarly exposed HpD-treated cells (less than 60%). No significant differences were detected between the responses of the fibrosarcoma and the fibroblast cell lines. These characteristics of AlSPc suggest that it may prove to be a useful photosensitizer for PDT of cancer and this possibility is discussed.
Full text
PDF




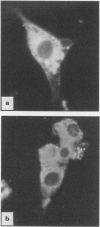
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J. Development of 3T3-like lines from Balb-c mouse embryo cultures: transformation susceptibility to SV40. J Cell Physiol. 1968 Oct;72(2):141–148. doi: 10.1002/jcp.1040720208. [DOI] [PubMed] [Google Scholar]
- Ben-Hur E., Rosenthal I. Photosensitized inactivation of Chinese hamster cells by phthalocyanines. Photochem Photobiol. 1985 Aug;42(2):129–133. doi: 10.1111/j.1751-1097.1985.tb01550.x. [DOI] [PubMed] [Google Scholar]
- Ben-Hur E., Rosenthal I. The phthalocyanines: a new class of mammalian cells photosensitizers with a potential for cancer phototherapy. Int J Radiat Biol Relat Stud Phys Chem Med. 1985 Feb;47(2):145–147. doi: 10.1080/09553008514550211. [DOI] [PubMed] [Google Scholar]
- Benson R. C., Jr, Farrow G. M., Kinsey J. H., Cortese D. A., Zincke H., Utz D. C. Detection and localization of In situ carcinoma of the bladder with hematoporphyrin derivative. Mayo Clin Proc. 1982 Sep;57(9):548–555. [PubMed] [Google Scholar]
- Berenbaum M. C., Bonnett R., Scourides P. A. In vivo biological activity of the components of haematoporphyrin derivative. Br J Cancer. 1982 Apr;45(4):571–581. doi: 10.1038/bjc.1982.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugelski P. J., Porter C. W., Dougherty T. J. Autoradiographic distribution of hematoporphyrin derivative in normal and tumor tissue of the mouse. Cancer Res. 1981 Nov;41(11 Pt 1):4606–4612. [PubMed] [Google Scholar]
- Carruth J. A., McKenzie A. L. Preliminary report of a pilot study of photoradiation therapy for the treatment of superficial malignancies of the skin, head and neck. Eur J Surg Oncol. 1985 Mar;11(1):47–50. [PubMed] [Google Scholar]
- Dahlman A., Wile A. G., Burns R. G., Mason G. R., Johnson F. M., Berns M. W. Laser photoradiation therapy of cancer. Cancer Res. 1983 Jan;43(1):430–434. [PubMed] [Google Scholar]
- Dougherty T. J. Hematoporphyrin derivative for detection and treatment of cancer. J Surg Oncol. 1980;15(3):209–210. doi: 10.1002/jso.2930150303. [DOI] [PubMed] [Google Scholar]
- Dougherty T. J., Kaufman J. E., Goldfarb A., Weishaupt K. R., Boyle D., Mittleman A. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978 Aug;38(8):2628–2635. [PubMed] [Google Scholar]
- Dougherty T. J., Lawrence G., Kaufman J. H., Boyle D., Weishaupt K. R., Goldfarb A. Photoradiation in the treatment of recurrent breast carcinoma. J Natl Cancer Inst. 1979 Feb;62(2):231–237. [PubMed] [Google Scholar]
- Evensen J. F., Sommer S., Moan J., Christensen T. Tumor-localizing and photosensitizing properties of the main components of hematoporphyrin derivative. Cancer Res. 1984 Feb;44(2):482–486. [PubMed] [Google Scholar]
- Forbes I. J., Cowled P. A., Leong A. S., Ward A. D., Black R. B., Blake A. J., Jacka F. J. Phototherapy of human tumours using haematoporphyrin derivative. Med J Aust. 1980 Nov 1;2(9):489–493. doi: 10.5694/j.1326-5377.1980.tb100708.x. [DOI] [PubMed] [Google Scholar]
- Gomer C. J., Smith D. M. Photoinactivation of Chinese hamster cells by hematoporphyrin derivative and red light. Photochem Photobiol. 1980 Sep;32(3):341–348. doi: 10.1111/j.1751-1097.1980.tb03772.x. [DOI] [PubMed] [Google Scholar]
- Gregorie H. B., Jr, Horger E. O., Ward J. L., Green J. F., Richards T., Robertson H. C., Jr, Stevenson T. B. Hematoporphyrin-derivative fluorescence in malignant neoplasms. Ann Surg. 1968 Jun;167(6):820–828. doi: 10.1097/00000658-196806000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greig R. G., Koestler T. P., Trainer D. L., Corwin S. P., Miles L., Kline T., Sweet R., Yokoyama S., Poste G. Tumorigenic and metastatic properties of "normal" and ras-transfected NIH/3T3 cells. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3698–3701. doi: 10.1073/pnas.82.11.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha X. W., Sun X. M., Xie J. G., Fan X. J., Zhang Y. H., Mei Q. C., Shen H., Xu S. L., Zhang R. G. Clinical use of hematoporphyrin derivative in malignant tumors. Chin Med J (Engl) 1983 Oct;96(10):754–758. [PubMed] [Google Scholar]
- Henderson B. W., Bellnier D. A., Ziring B., Dougherty T. J. Aspects of the cellular uptake and retention of hematoporphyrin derivative and their correlation with the biological response to PRT in vitro. Adv Exp Med Biol. 1983;160:129–138. doi: 10.1007/978-1-4684-4406-3_13. [DOI] [PubMed] [Google Scholar]
- Henderson B. W., Waldow S. M., Mang T. S., Potter W. R., Malone P. B., Dougherty T. J. Tumor destruction and kinetics of tumor cell death in two experimental mouse tumors following photodynamic therapy. Cancer Res. 1985 Feb;45(2):572–576. [PubMed] [Google Scholar]
- Kelly J. F., Snell M. E. Hematoporphyrin derivative: a possible aid in the diagnosis and therapy of carcinoma of the bladder. J Urol. 1976 Feb;115(2):150–151. doi: 10.1016/s0022-5347(17)59108-9. [DOI] [PubMed] [Google Scholar]
- Kessel D., Cheng M. L. Biological and biophysical properties of the tumor-localizing component of hematoporphyrin derivative. Cancer Res. 1985 Jul;45(7):3053–3057. [PMC free article] [PubMed] [Google Scholar]
- Kinsey J. H., Cortese D. A., Moses H. L., Ryan R. J., Branum E. L. Photodynamic effect of hematoporphyrin derivative as a function of optical spectrum and incident energy density. Cancer Res. 1981 Dec;41(12 Pt 1):5020–5026. [PubMed] [Google Scholar]
- Kripke M. L., Gruys E., Fidler I. J. Metastatic heterogeneity of cells from an ultraviolet light-induced murine fibrosarcoma of recent origin. Cancer Res. 1978 Sep;38(9):2962–2967. [PubMed] [Google Scholar]
- LIPSON R. L., BALDES E. J., OLSEN A. M. The use of a derivative of hematoporhyrin in tumor detection. J Natl Cancer Inst. 1961 Jan;26:1–11. [PubMed] [Google Scholar]
- Leonard J. R., Beck W. L. Hematoporphyrin fluorescence: an aid in diagnosis of malignant neoplasms. Laryngoscope. 1971 Mar;81(3):365–372. doi: 10.1288/00005537-197103000-00003. [DOI] [PubMed] [Google Scholar]
- Moan J., McGhie J., Jacobsen P. B. Photodynamic effects on cells in vitro exposed to hematoporphyrin derivative and light. Photochem Photobiol. 1983 Jun;37(6):599–604. doi: 10.1111/j.1751-1097.1983.tb04527.x. [DOI] [PubMed] [Google Scholar]
- Moan J., Pettersen E. O., Christensen T. The mechanism of photodynamic inactivation of human cells in vitro in the presence of haematoporphyrin. Br J Cancer. 1979 Apr;39(4):398–407. doi: 10.1038/bjc.1979.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Profio A. E., Doiron D. R. A feasibility study of the use of fluorescence bronchoscopy for localization of small lung tumours. Phys Med Biol. 1977 Sep;22(5):949–957. doi: 10.1088/0031-9155/22/5/014. [DOI] [PubMed] [Google Scholar]
- Rousseau J., Autenrieth D., van Lier J. E. Synthesis, tissue distribution and tumor uptake of [99Tc]tetrasulfophthalocyanine. Int J Appl Radiat Isot. 1983 Mar;34(3):571–579. doi: 10.1016/0020-708x(83)90058-3. [DOI] [PubMed] [Google Scholar]
- Selman S. H., Kreimer-Birnbaum M., Klaunig J. E., Goldblatt P. J., Keck R. W., Britton S. L. Blood flow in transplantable bladder tumors treated with hematoporphyrin derivative and light. Cancer Res. 1984 May;44(5):1924–1927. [PubMed] [Google Scholar]
- Weishaupt K. R., Gomer C. J., Dougherty T. J. Identification of singlet oxygen as the cytotoxic agent in photoinactivation of a murine tumor. Cancer Res. 1976 Jul;36(7 Pt 1):2326–2329. [PubMed] [Google Scholar]