Abstract
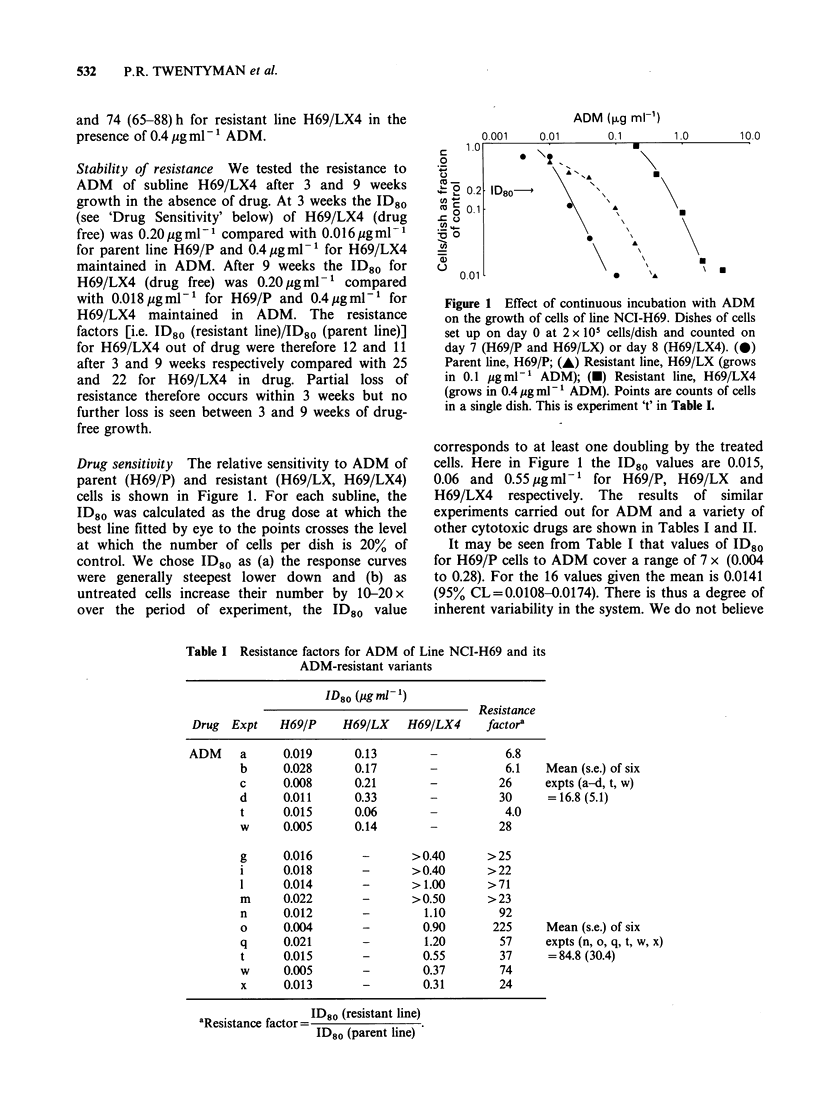
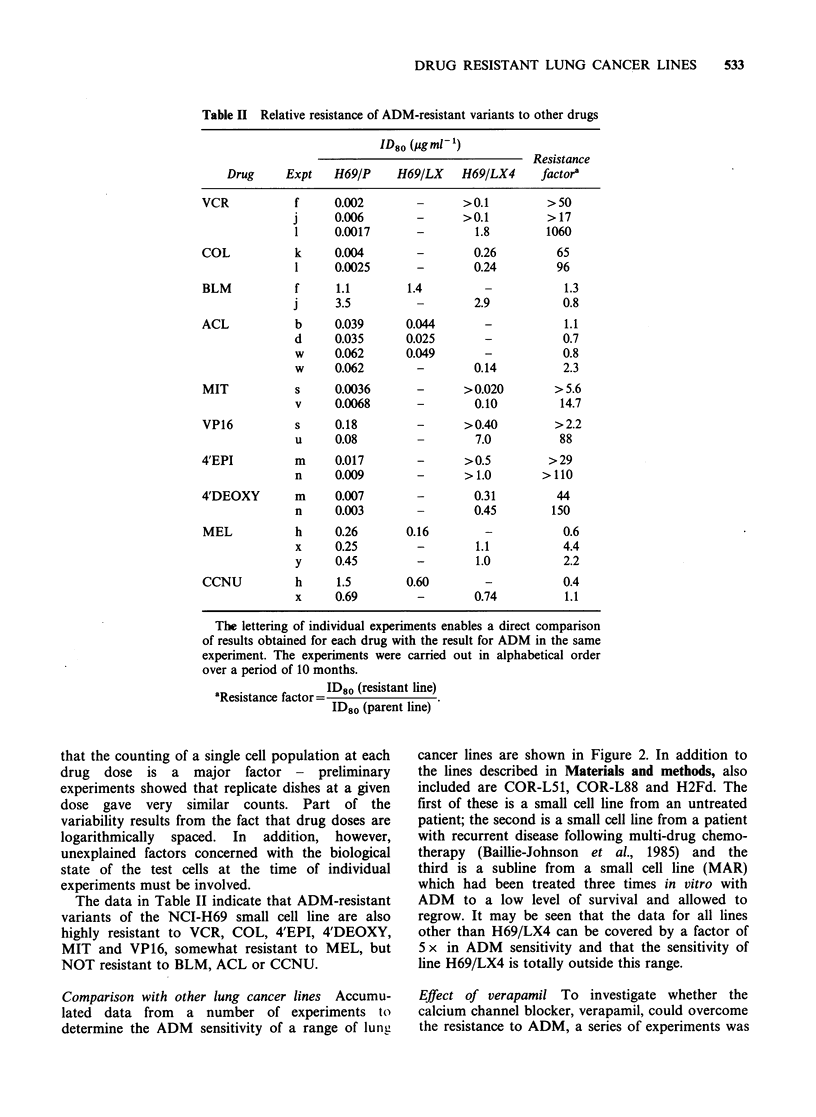
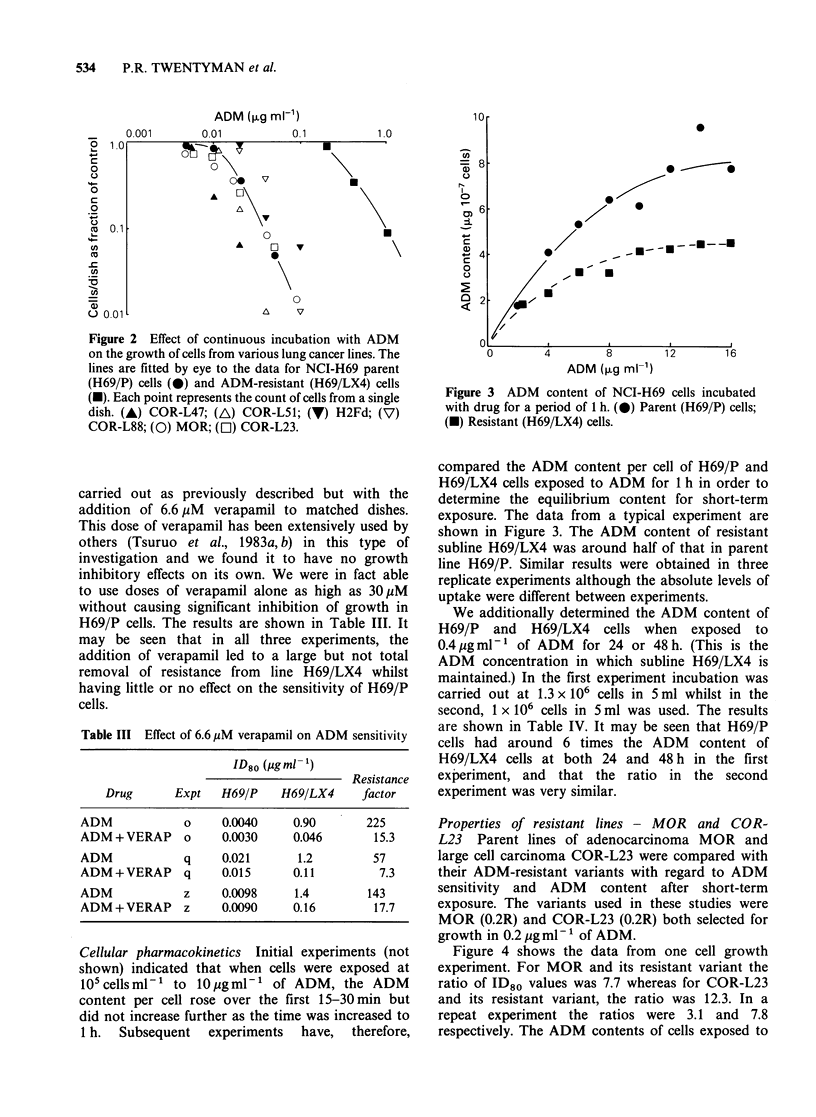
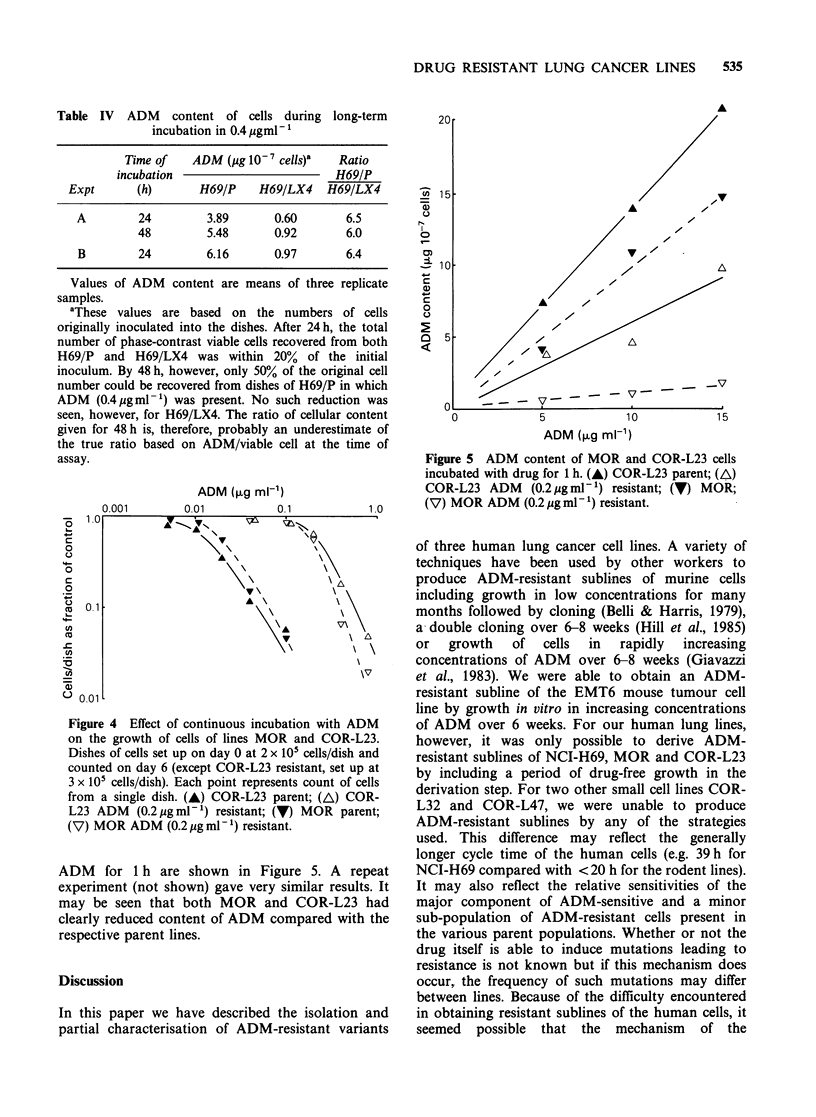
We have produced adriamycin (ADM)-resistant variants of the human lung cancer cell lines NCI-H69 (small cell), MOR (adenocarcinoma) and COR-L23 (large cell) but have failed to produce resistant variants of two other small cell lines. In each case, the derivation protocol took 7-9 months and included a period of drug-free growth. All three resistant lines show reduced cellular content of ADM after 1 h exposure when compared with their controls. During prolonged incubation of control and resistant NCI-H69 cells in 0.4 microgram ml-1 ADM, the ADM content of resistant cells was 6-7 times lower than that of control cells. The ratio of ADM doses to suppress growth of the two lines, however, was in the range of 40-200X. The ADM-resistant variant of NCI-H69 was also resistant to vincristine, colchicine, VP16, mitozantrone, 4' epiadriamycin and 4' deoxyadriamycin, somewhat resistant to melphalan but not resistant to aclacinomycin A, bleomycin of CCNU. The resistance to ADM could be partially overcome by the use of verapamil, an inhibitor of calcium transport.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babson J. R., Abell N. S., Reed D. J. Protective role of the glutathione redox cycle against adriamycin-mediated toxicity in isolated hepatocytes. Biochem Pharmacol. 1981 Aug 15;30(16):2299–2304. doi: 10.1016/0006-2952(81)90102-7. [DOI] [PubMed] [Google Scholar]
- Baillie-Johnson H., Twentyman P. R., Fox N. E., Walls G. A., Workman P., Watson J. V., Johnson N., Reeve J. G., Bleehen N. M. Establishment and characterisation of cell lines from patients with lung cancer (predominantly small cell carcinoma). Br J Cancer. 1985 Oct;52(4):495–504. doi: 10.1038/bjc.1985.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bech-Hansen N. T., Till J. E., Ling V. Pleiotropic phenotype of colchicine-resistant CHO cells: cross-resistance and collateral sensitivity. J Cell Physiol. 1976 May;88(1):23–31. doi: 10.1002/jcp.1040880104. [DOI] [PubMed] [Google Scholar]
- Beck W. T. Vinca alkaloid-resistant phenotype in cultured human leukemic lymphoblasts. Cancer Treat Rep. 1983 Oct;67(10):875–882. [PubMed] [Google Scholar]
- Belli J. A., Harris J. R. Adriamycin resistance and radiation response. Int J Radiat Oncol Biol Phys. 1979 Aug;5(8):1231–1234. doi: 10.1016/0360-3016(79)90644-8. [DOI] [PubMed] [Google Scholar]
- Biedler J. L., Chang T. D., Meyers M. B., Peterson R. H., Spengler B. A. Drug resistance in Chinese hamster lung and mouse tumor cells. Cancer Treat Rep. 1983 Oct;67(10):859–867. [PubMed] [Google Scholar]
- Dano K. Active outward transport of daunomycin in resistant Ehrlich ascites tumor cells. Biochim Biophys Acta. 1973 Oct 25;323(3):466–483. doi: 10.1016/0005-2736(73)90191-0. [DOI] [PubMed] [Google Scholar]
- Dano K. Cross resistance between vinca alkaloids and anthracyclines in Ehrlich ascites tumor in vivo. Cancer Chemother Rep. 1972 Dec;56(6):701–708. [PubMed] [Google Scholar]
- Elliott E. M., Ling V. Selection and characterization of Chinese hamster ovary cell mutants resistant to melphalan (L-phenylalanine mustard). Cancer Res. 1981 Feb;41(2):393–400. [PubMed] [Google Scholar]
- Giavazzi R., Scholar E., Hart I. R. Isolation and preliminary characterization of an Adriamycin-resistant murine fibrosarcoma cell line. Cancer Res. 1983 May;43(5):2216–2222. [PubMed] [Google Scholar]
- Hill B. T., Dennis L. Y., Li X. T., Whelan R. D. Identification of anthracycline analogues with enhanced cytotoxicity and lack of cross-resistance to adriamycin using a series of mammalian cell lines in vitro. Cancer Chemother Pharmacol. 1985;14(3):194–201. doi: 10.1007/BF00258115. [DOI] [PubMed] [Google Scholar]
- Inaba M., Johnson R. K. Uptake and retention of adriamycin and daunorubicin by sensitive and anthracycline-resistant sublines of P388 leukemia. Biochem Pharmacol. 1978;27(17):2123–2130. doi: 10.1016/0006-2952(78)90284-8. [DOI] [PubMed] [Google Scholar]
- Inaba M., Kobayashi H., Sakurai Y., Johnson R. K. Active efflux of daunorubicin and adriamycin in sensitive and resistant sublines of P388 leukemia. Cancer Res. 1979 Jun;39(6 Pt 1):2200–2203. [PubMed] [Google Scholar]
- Johnson R. K., Ovejera A. A., Goldin A. Activity of anthracyclines against an adriamycin (NSC-123127)-resistant subline of P388 leukemia with special emphasis on cinerubin A (NSC-18334). Cancer Treat Rep. 1976 Jan;60(1):99–102. [PubMed] [Google Scholar]
- Kartner N., Riordan J. R., Ling V. Cell surface P-glycoprotein associated with multidrug resistance in mammalian cell lines. Science. 1983 Sep 23;221(4617):1285–1288. doi: 10.1126/science.6137059. [DOI] [PubMed] [Google Scholar]
- Kessel D., Botterill V., Wodinsky I. Uptake and retention of daunomycin by mouse leukemic cells as factors in drug response. Cancer Res. 1968 May;28(5):938–941. [PubMed] [Google Scholar]
- Mungikar A., Chitnis M., Gothoskar B. Mixed-function oxidase enzymes in adriamycin-sensitive and resistant sublines of P-388 leukemia. Chem Biol Interact. 1981 Apr;35(1):119–124. doi: 10.1016/0009-2797(81)90067-3. [DOI] [PubMed] [Google Scholar]
- Nishimura T., Muto K., Tanaka N. Drug sensitivity of an adriamycin-resistant mutant subline of mouse lymphoblastoma L5178Y cells. J Antibiot (Tokyo) 1978 May;31(5):493–495. doi: 10.7164/antibiotics.31.493. [DOI] [PubMed] [Google Scholar]
- Roninson I. B., Abelson H. T., Housman D. E., Howell N., Varshavsky A. Amplification of specific DNA sequences correlates with multi-drug resistance in Chinese hamster cells. Nature. 1984 Jun 14;309(5969):626–628. doi: 10.1038/309626a0. [DOI] [PubMed] [Google Scholar]
- Schabel F. M., Jr, Skipper H. E., Trader M. W., Laster W. R., Jr, Griswold D. P., Jr, Corbett T. H. Establishment of cross-resistance profiles for new agents. Cancer Treat Rep. 1983 Oct;67(10):905–922. [PubMed] [Google Scholar]
- Schwartz H. S. A fluorometric assay for daunomycin and adriamycin in animal tissues. Biochem Med. 1973 Jun;7(3):396–404. doi: 10.1016/0006-2944(73)90060-4. [DOI] [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Nojiri M., Tsukagoshi S., Sakurai Y. Circumvention of vincristine and Adriamycin resistance in vitro and in vivo by calcium influx blockers. Cancer Res. 1983 Jun;43(6):2905–2910. [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Increased accumulation of vincristine and adriamycin in drug-resistant P388 tumor cells following incubation with calcium antagonists and calmodulin inhibitors. Cancer Res. 1982 Nov;42(11):4730–4733. [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Potentiation of vincristine and Adriamycin effects in human hemopoietic tumor cell lines by calcium antagonists and calmodulin inhibitors. Cancer Res. 1983 May;43(5):2267–2272. [PubMed] [Google Scholar]


