Abstract
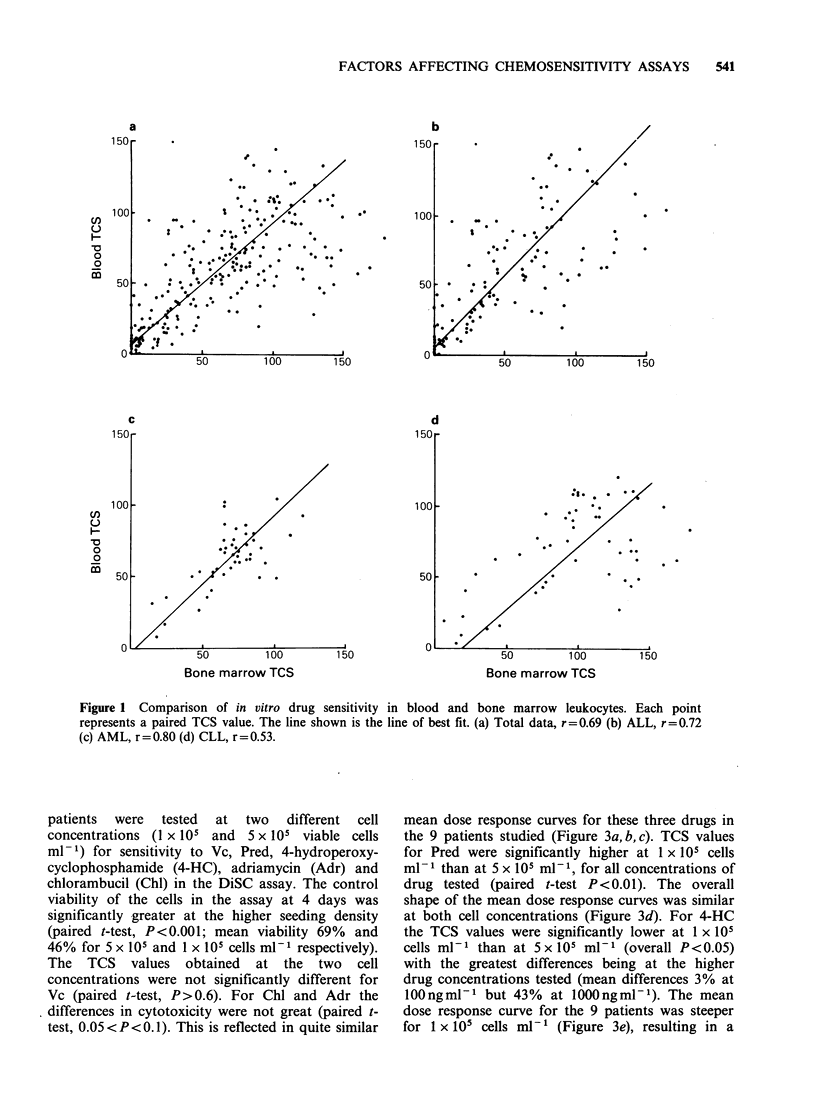
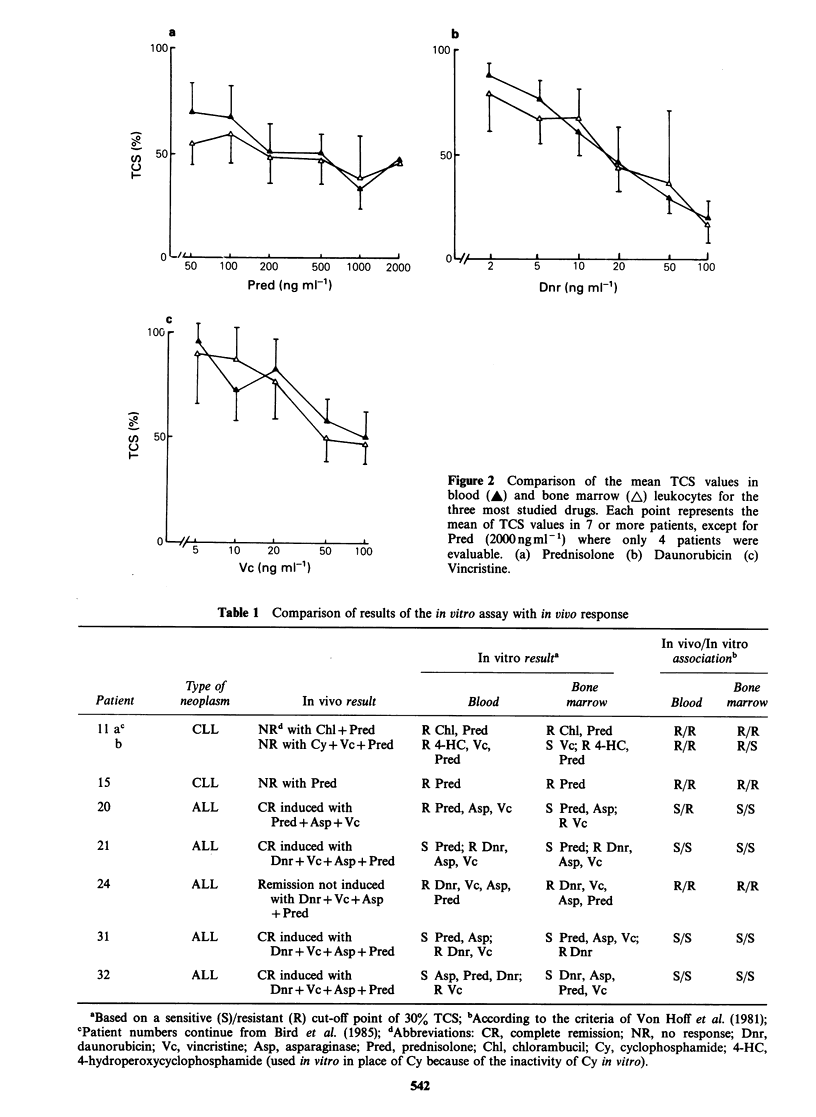
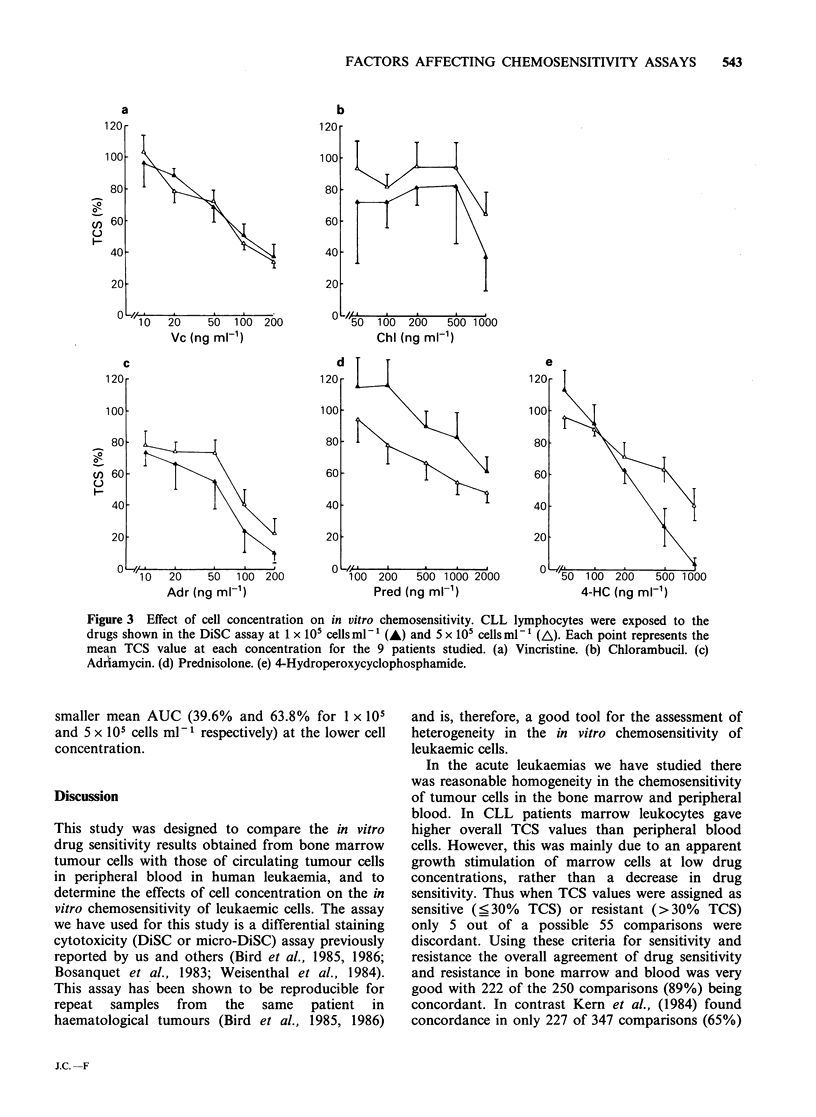
The Differential Staining Cytotoxicity (DiSC) assay has been used to study the effects of sample source and cell concentration on the in vitro chemosensitivity of haematological malignancies. The chemosensitivity of blood and bone marrow samples was significantly associated (P less than 0.001) in 12 cases where both were tested simultaneously. In 8 of the cases, where the in vitro result could be compared with clinical response, the in vitro and in vivo chemosensitivity was in agreement in 7, for both blood and bone marrow samples. The in vitro chemosensitivity of chronic lymphocytic leukaemia blood lymphocytes was dependent on the cell concentration for 4 out of 5 drugs tested. A five fold reduction in cell number resulted in a significantly greater cell kill with 4-hydroperoxycyclophosphamide, a greater cell kill (not significant) with chlorambucil and adriamycin, and a significantly lower cell kill with prednisolone. The cell concentration did not affect vincristine cytotoxicity. These results suggest that sample source is not an important consideration for the in vitro chemosensitivity of leukaemias, but that the cell concentration tested should not be varied from assay to assay if the results are to be used for comparative purposes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird M. C., Bosanquet A. G., Gilby E. D. In vitro determination of tumour chemosensitivity in haematological malignancies. Hematol Oncol. 1985 Jan-Mar;3(1):1–10. doi: 10.1002/hon.2900030102. [DOI] [PubMed] [Google Scholar]
- Bosanquet A. G., Bird M. C., Price W. J., Gilby E. D. An assessment of a short-term tumour chemosensitivity assay in chronic lymphocytic leukaemia. Br J Cancer. 1983 Jun;47(6):781–789. doi: 10.1038/bjc.1983.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers S. H., Bleehen N. M., Watson J. V. Effect of cell density on intracellular adriamycin concentration and cytotoxicity in exponential and plateau phase EMT6 cells. Br J Cancer. 1984 Mar;49(3):301–306. doi: 10.1038/bjc.1984.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durie B. G. Experimental approaches to drug testing and clonogenic growth: results in multiple myeloma and acute myelogenous leukemia. Recent Results Cancer Res. 1984;94:93–101. doi: 10.1007/978-3-642-82295-7_10. [DOI] [PubMed] [Google Scholar]
- Herve P., Tamayo E., Peters A. Autologous stem cell grafting in acute myeloid leukaemia: technical approach of marrow incubation in vitro with pharmacological agents (prerequisite for clinical applications). Br J Haematol. 1983 Apr;53(4):683–685. doi: 10.1111/j.1365-2141.1983.tb07320.x. [DOI] [PubMed] [Google Scholar]
- Hill B. T. Cancer chemotherapy. The relevance of certain concepts of cell cycle kinetics. Biochim Biophys Acta. 1978 Dec 11;516(4):389–417. doi: 10.1016/0304-419x(78)90018-5. [DOI] [PubMed] [Google Scholar]
- Holbrook N. J., Bloomfield C. D., Munck A. Stabilization of labile glucocorticoid-receptor complexes from acute nonlymphocytic leukemia cells by a factor from chronic lymphocytic leukemia cells. Cancer Res. 1984 Jan;44(1):407–414. [PubMed] [Google Scholar]
- Körbling M., Hess A. D., Tutschka P. J., Kaizer H., Colvin M. O., Santos G. W. 4-hydroperoxycyclophosphamide: a model for eliminating residual human tumour cells and T-lymphocytes from the bone marrow graft. Br J Haematol. 1982 Sep;52(1):89–96. doi: 10.1111/j.1365-2141.1982.tb03864.x. [DOI] [PubMed] [Google Scholar]
- Norton L., Simon R. Tumor size, sensitivity to therapy, and design of treatment schedules. Cancer Treat Rep. 1977 Oct;61(7):1307–1317. [PubMed] [Google Scholar]
- Ozols R. F., Hogan W. M., Young R. C. Direct cloning of human ovarian cancer in soft agar: clinical limitations and pharmacologic applications. Recent Results Cancer Res. 1984;94:41–50. doi: 10.1007/978-3-642-82295-7_5. [DOI] [PubMed] [Google Scholar]
- Sanfilippo O., Daidone M. G., Zaffaroni N., Silvestrini R. Development of a nucleotide precursor incorporation assay for testing drug sensitivity of human tumors. Recent Results Cancer Res. 1984;94:127–139. doi: 10.1007/978-3-642-82295-7_13. [DOI] [PubMed] [Google Scholar]
- Schlag P., Schreml W. Heterogeneity in growth pattern and drug sensitivity of primary tumor and metastases in the human tumor colony-forming assay. Cancer Res. 1982 Oct;42(10):4086–4089. [PubMed] [Google Scholar]
- Von Hoff D. D., Casper J., Bradley E., Sandbach J., Jones D., Makuch R. Association between human tumor colony-forming assay results and response of an individual patient's tumor to chemotherapy. Am J Med. 1981 May;70(5):1027–1041. doi: 10.1016/0002-9343(81)90859-7. [DOI] [PubMed] [Google Scholar]
- Weisenthal L. M., Shoemaker R. H., Marsden J. A., Dill P. L., Baker J. A., Moran E. M. In vitro chemosensitivity assay based on the concept of total tumor cell kill. Recent Results Cancer Res. 1984;94:161–173. doi: 10.1007/978-3-642-82295-7_16. [DOI] [PubMed] [Google Scholar]


