Abstract
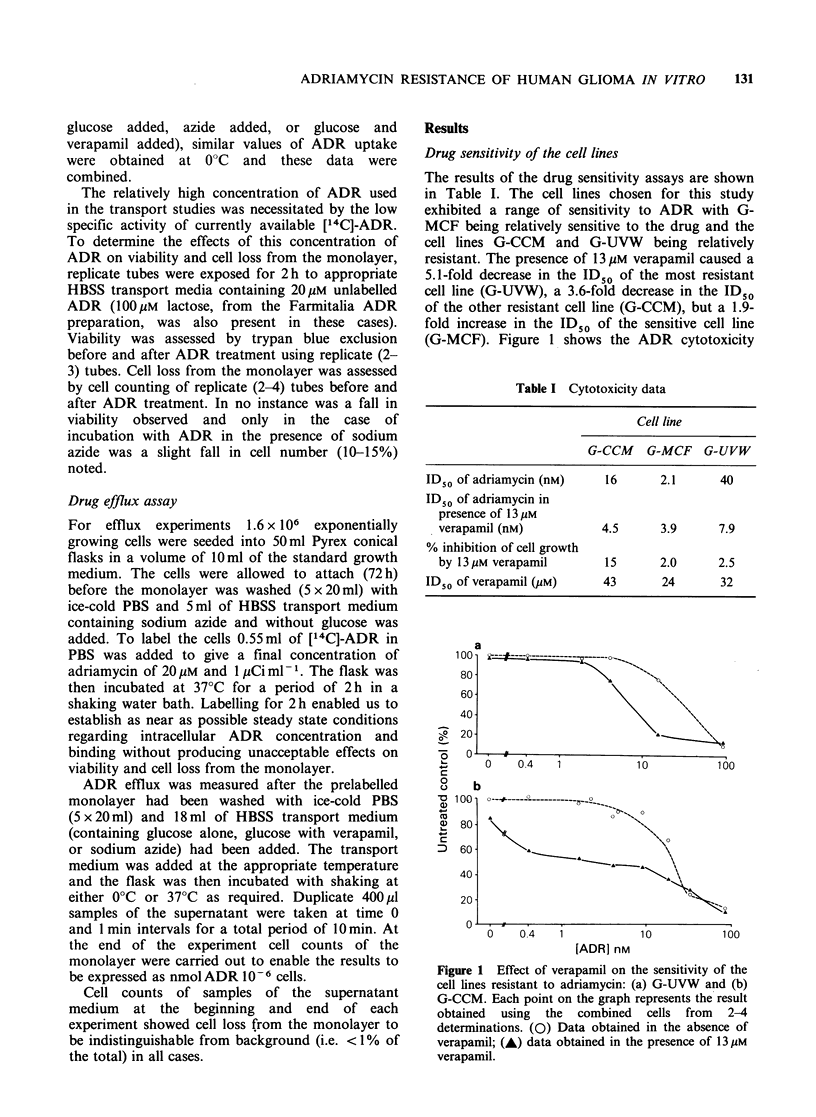
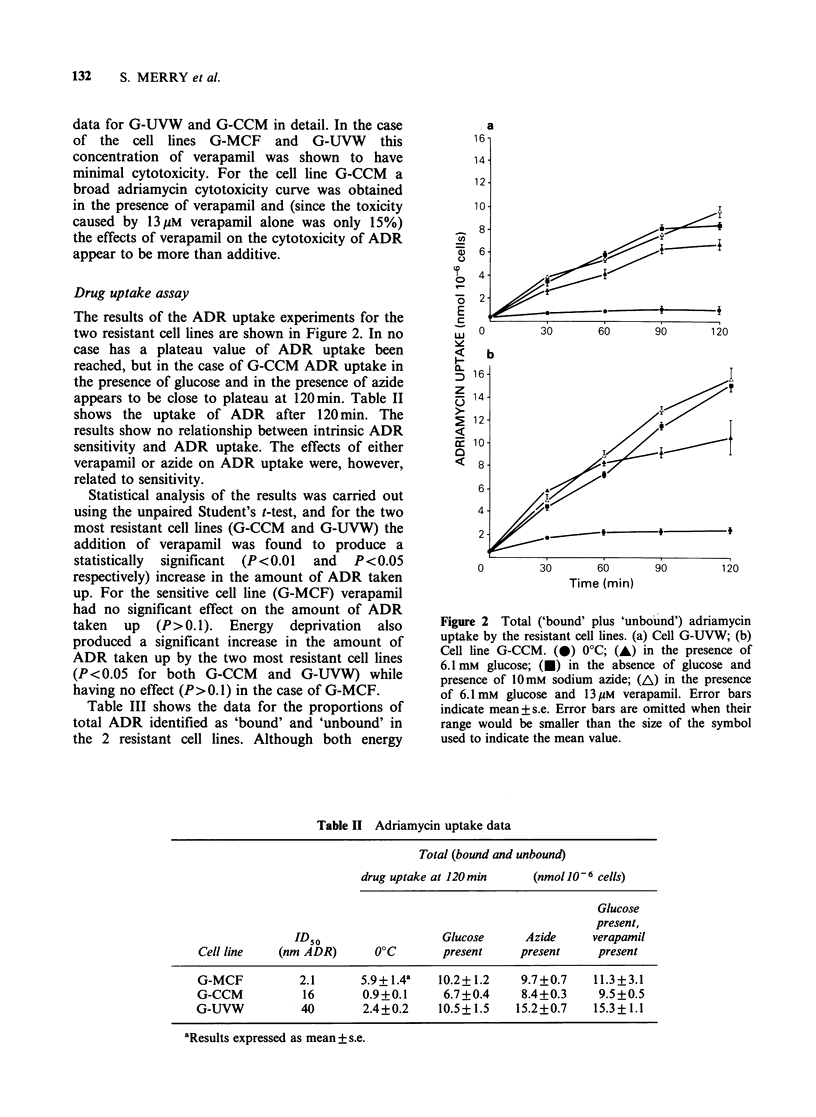
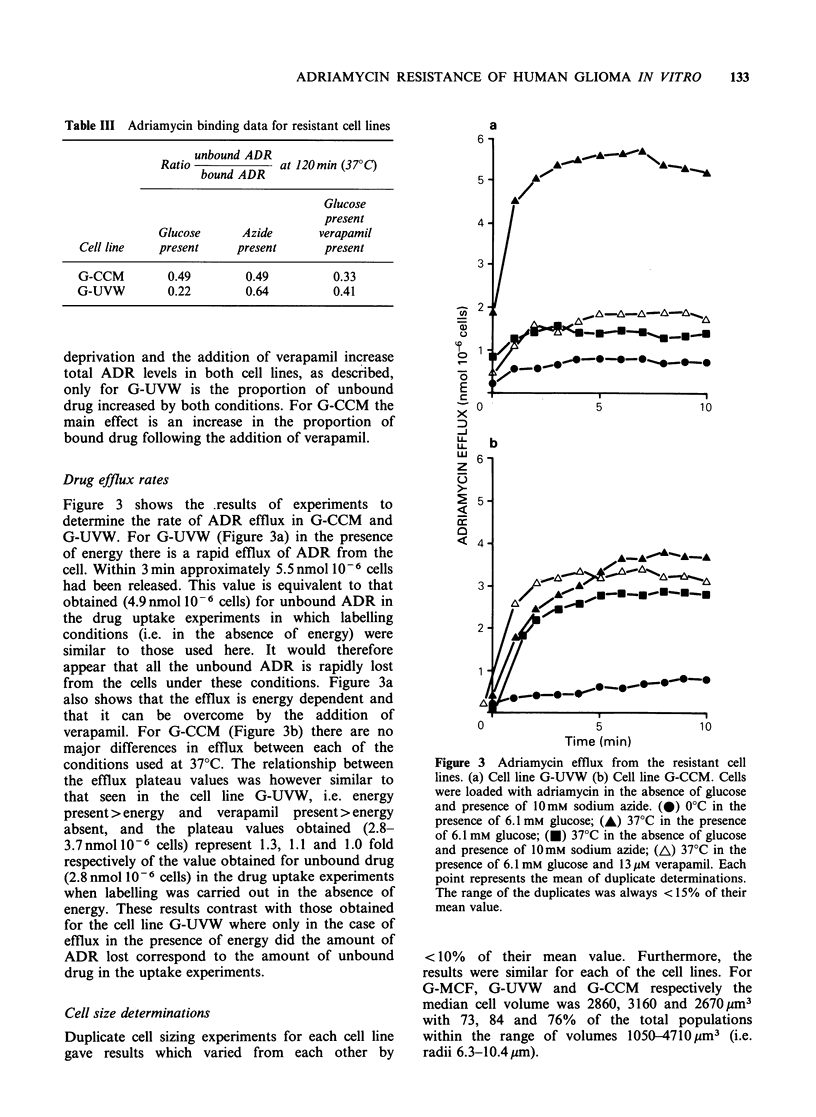
We have investigated the mechanism of resistance to adriamycin (ADR) of 3 human glioma cell lines in culture. The cell lines had different inherent sensitivities to ADR. Verapamil increased the ADR sensitivities of the 2 most resistant cell lines (G-UVW and G-CCM) by up to 5-fold. This effect was not seen in a sensitive cell line (G-MCF). Although the accumulation of ADR in the 3 cell lines was not related to inherent sensitivity, energy deprivation or the addition of verapamil produced an increase (up to 46%) in net uptake for both G-UVW and G-CCM, but not for G-MCF. For G-UVW the ADR efflux data were consistent with an energy-dependent ADR efflux mechanism which could be inhibited by verapamil. A similar mechanism was not found for G-CCM. In this cell line verapamil may act by increasing intracellular ADR binding. These data indicate that, while inherent resistance to ADR may be multifactorial, one possible mechanism of resistance in human glioma may involve changes in drug accumulation and/or binding as has been seen in animals models. A potential clinical role for verapamil in overcoming drug resistance in human solid tumours is also indicated.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck W. T. Vinca alkaloid-resistant phenotype in cultured human leukemic lymphoblasts. Cancer Treat Rep. 1983 Oct;67(10):875–882. [PubMed] [Google Scholar]
- Chabner B. A., Clendeninn N. J., Curt G. A. Symposium on cellular resistance to anticancer drugs. Introduction. Cancer Treat Rep. 1983 Oct;67(10):855–857. [PubMed] [Google Scholar]
- Chang B. K., Gregory J. A. Comparison of the cellular pharmacology of doxorubicin in resistant and sensitive models of pancreatic cancer. Cancer Chemother Pharmacol. 1985;14(2):132–134. doi: 10.1007/BF00434351. [DOI] [PubMed] [Google Scholar]
- Ganapathi R., Grabowski D. Enhancement of sensitivity to adriamycin in resistant P388 leukemia by the calmodulin inhibitor trifluoperazine. Cancer Res. 1983 Aug;43(8):3696–3699. [PubMed] [Google Scholar]
- Ganapathi R., Grabowski D., Turinic R., Valenzuela R. Correlation between potency of calmodulin inhibitors and effects on cellular levels and cytotoxic activity of doxorubicin (adriamycin) in resistant P388 mouse leukemia cells. Eur J Cancer Clin Oncol. 1984 Jun;20(6):799–806. doi: 10.1016/0277-5379(84)90219-0. [DOI] [PubMed] [Google Scholar]
- Kaye S., Merry S. Tumour cell resistance to anthracyclines--a review. Cancer Chemother Pharmacol. 1985;14(2):96–103. doi: 10.1007/BF00434344. [DOI] [PubMed] [Google Scholar]
- Kessel D., Wilberding C. Anthracycline resistance in P388 murine leukemia and its circumvention by calcium antagonists. Cancer Res. 1985 Apr;45(4):1687–1691. [PubMed] [Google Scholar]
- Merry S., Kaye S. B., Freshney R. I. Cross-resistance to cytotoxic drugs in human glioma cell lines in culture. Br J Cancer. 1984 Dec;50(6):831–835. doi: 10.1038/bjc.1984.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramu A., Fuks Z., Gatt S., Glaubiger D. Reversal of acquired resistance to doxorubicin in P388 murine leukemia cells by perhexiline maleate. Cancer Res. 1984 Jan;44(1):144–148. [PubMed] [Google Scholar]
- Rogan A. M., Hamilton T. C., Young R. C., Klecker R. W., Jr, Ozols R. F. Reversal of adriamycin resistance by verapamil in human ovarian cancer. Science. 1984 Jun 1;224(4652):994–996. doi: 10.1126/science.6372095. [DOI] [PubMed] [Google Scholar]
- Shoemaker R. H., Curt G. A., Carney D. N. Evidence for multidrug-resistant cells in human tumor cell populations. Cancer Treat Rep. 1983 Oct;67(10):883–888. [PubMed] [Google Scholar]
- Slater L. M., Murray S. L., Wetzel M. W., Wisdom R. M., DuVall E. M. Verapamil restoration of daunorubicin responsiveness in daunorubicin-resistant Ehrlich ascites carcinoma. J Clin Invest. 1982 Nov;70(5):1131–1134. doi: 10.1172/JCI110702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Naganuma K., Tsukagoshi S., Sakurai Y. Promotion by verapamil of vincristine responsiveness in tumor cell lines inherently resistant to the drug. Cancer Res. 1983 Feb;43(2):808–813. [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Nojiri M., Tsukagoshi S., Sakurai Y. Circumvention of vincristine and Adriamycin resistance in vitro and in vivo by calcium influx blockers. Cancer Res. 1983 Jun;43(6):2905–2910. [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Increased accumulation of vincristine and adriamycin in drug-resistant P388 tumor cells following incubation with calcium antagonists and calmodulin inhibitors. Cancer Res. 1982 Nov;42(11):4730–4733. [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res. 1981 May;41(5):1967–1972. [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Potentiation of vincristine and Adriamycin effects in human hemopoietic tumor cell lines by calcium antagonists and calmodulin inhibitors. Cancer Res. 1983 May;43(5):2267–2272. [PubMed] [Google Scholar]
- Yalowich J. C., Ross W. E. Potentiation of etoposide-induced DNA damage by calcium antagonists in L1210 cells in vitro. Cancer Res. 1984 Aug;44(8):3360–3365. [PubMed] [Google Scholar]


