Abstract
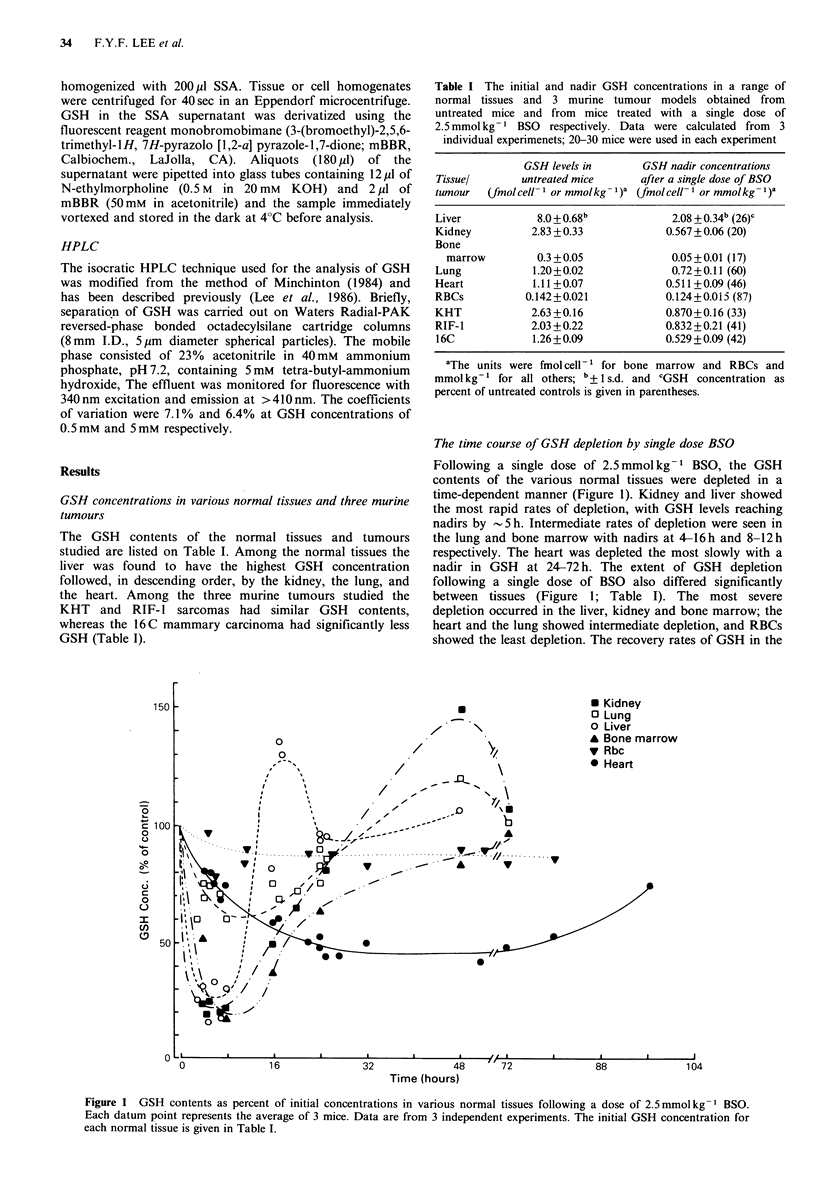
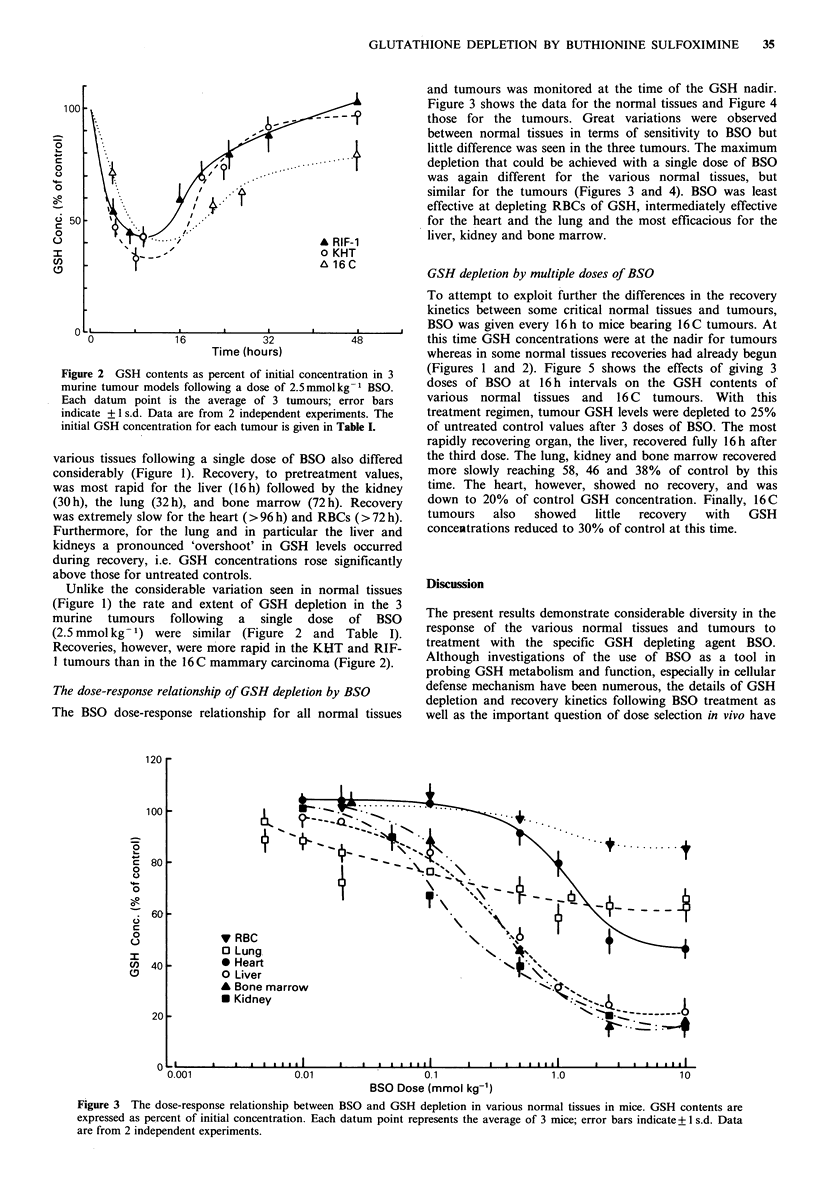
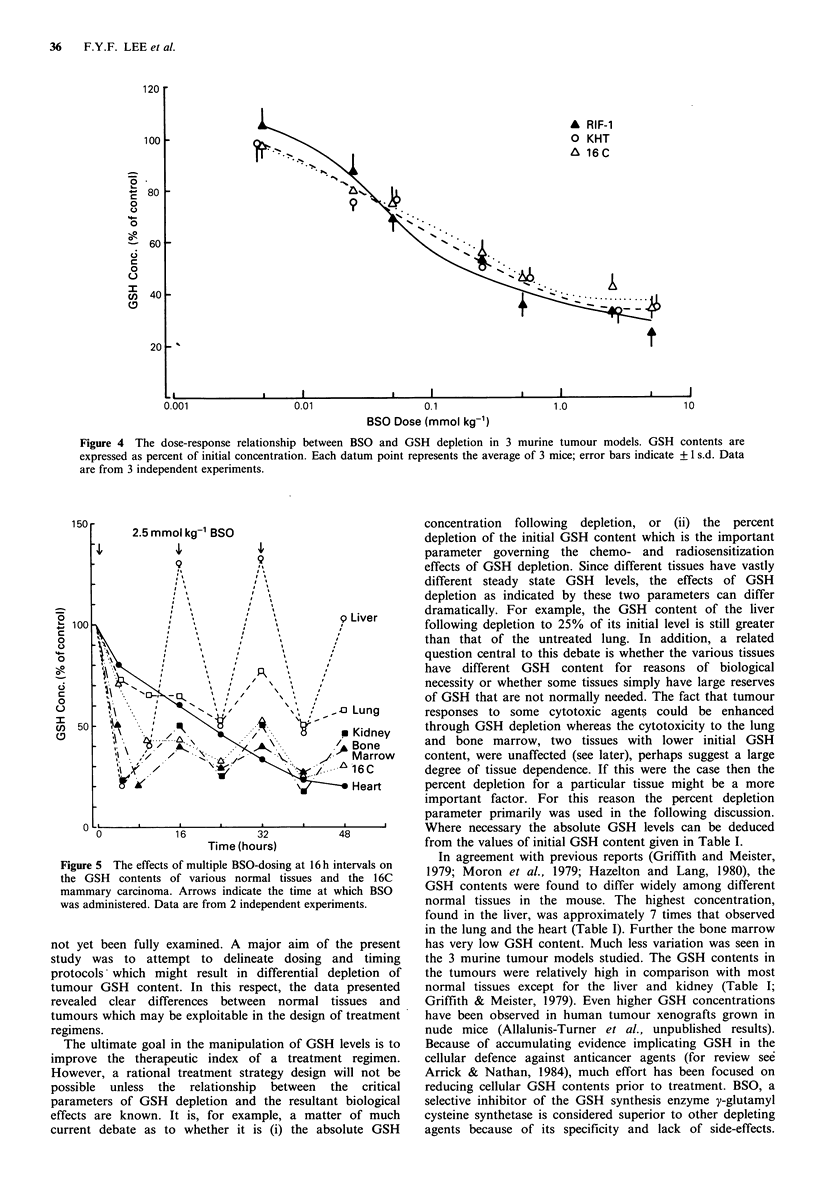
We have investigated in detail the effects of buthionine sulfoximine (BSO), a selective glutathione (GSH) depleting agent, on the GSH contents of a number of normal tissues and three experimental tumours in mice. Significant variations in the rate and degree of GSH depletion and recovery were observed among the normal tissues. Following a dose of 2.5 mmol kg-1 BSO, GSH nadirs were reached by approximately 5 h for the liver and kidney, 8 h for the lung and bone marrow, 12 h for red blood cells (RBCs) and by 24 h for the heart. The degree of depletion was greatest for the kidney (80%), liver (74%) and bone marrow (83%), intermediate for the heart (54%) and lung (40%), and least for RBCs (13%). Recovery of GSH content was fastest for the liver followed in descending order by the kidney, the lung, the bone marrow, RBCs and the heart. In contrast, the rate and extent of GSH depletion and recovery showed considerably less variation among the 3 murine tumours. In the tumours GSH nadirs were reached by 10-12 h. The extent of depletion was about 55-65%. Recovery of GSH levels in the tumours required 48 h or more, a longer period than required by the liver, kidney and lung but shorter than that needed for the bone marrow, heart and RBCs. Attempts to preferentially deplete tumour GSH by exploiting the differences in recovery rates between normal tissues and tumours were only partially successful. Multiple BSO dosing at 16 h intervals allowed the liver to recover between doses, but the recovery in the kidney, lung and bone marrow was only partial and no recovery was seen in the heart. Finally, dose-depletion relationship investigations showed that, with the exception of the lungs, GSH depletion could be achieved in tumours with doses of BSO lower than those required for normal tissues.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrick B. A., Nathan C. F. Glutathione metabolism as a determinant of therapeutic efficacy: a review. Cancer Res. 1984 Oct;44(10):4224–4232. [PubMed] [Google Scholar]
- Biaglow J. E., Clark E. P., Epp E. R., Morse-Guadio M., Varnes M. E., Mitchell J. B. Nonprotein thiols and the radiation response of A549 human lung carcinoma cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1983 Nov;44(5):489–495. doi: 10.1080/09553008314551491. [DOI] [PubMed] [Google Scholar]
- Corbett T. H., Griswold D. P., Jr, Roberts B. J., Peckham J. C., Schabel F. M., Jr Biology and therapeutic response of a mouse mammary adenocarcinoma (16/C) and its potential as a model for surgical adjuvant chemotherapy. Cancer Treat Rep. 1978 Oct;62(10):1471–1488. [PubMed] [Google Scholar]
- Crook T. R., Souhami R. L., Whyman G. D., McLean A. E. Glutathione depletion as a determinant of sensitivity of human leukemia cells to cyclophosphamide. Cancer Res. 1986 Oct;46(10):5035–5038. [PubMed] [Google Scholar]
- Green J. A., Vistica D. T., Young R. C., Hamilton T. C., Rogan A. M., Ozols R. F. Potentiation of melphalan cytotoxicity in human ovarian cancer cell lines by glutathione depletion. Cancer Res. 1984 Nov;44(11):5427–5431. [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Glutathione: interorgan translocation, turnover, and metabolism. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5606–5610. doi: 10.1073/pnas.76.11.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton T. C., Winker M. A., Louie K. G., Batist G., Behrens B. C., Tsuruo T., Grotzinger K. R., McKoy W. M., Young R. C., Ozols R. F. Augmentation of adriamycin, melphalan, and cisplatin cytotoxicity in drug-resistant and -sensitive human ovarian carcinoma cell lines by buthionine sulfoximine mediated glutathione depletion. Biochem Pharmacol. 1985 Jul 15;34(14):2583–2586. doi: 10.1016/0006-2952(85)90551-9. [DOI] [PubMed] [Google Scholar]
- Hazelton G. A., Lang C. A. Glutathione contents of tissues in the aging mouse. Biochem J. 1980 Apr 15;188(1):25–30. doi: 10.1042/bj1880025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallman R. F., Silini G., Van Putten L. M. Factors influencing the quantitative estimation of the in vivo survival of cells from solid tumors. J Natl Cancer Inst. 1967 Sep;39(3):539–549. [PubMed] [Google Scholar]
- Lefrak E. A., Pitha J., Rosenheim S., Gottlieb J. A. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer. 1973 Aug;32(2):302–314. doi: 10.1002/1097-0142(197308)32:2<302::aid-cncr2820320205>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Minchinton A. I. Measurements of glutathione and other thiols in cells and tissues: a simplified procedure based on the HPLC separation of monobromobimane derivatives of thiols. Int J Radiat Oncol Biol Phys. 1984 Sep;10(9):1503–1506. doi: 10.1016/0360-3016(84)90490-5. [DOI] [PubMed] [Google Scholar]
- Minchinton A. I., Rojas A., Smith K. A., Soranson J. A., Shrieve D. C., Jones N. R., Bremner J. C. Glutathione depletion in tissues after administration of buthionine sulphoximine. Int J Radiat Oncol Biol Phys. 1984 Aug;10(8):1261–1264. doi: 10.1016/0360-3016(84)90329-8. [DOI] [PubMed] [Google Scholar]
- Moron M. S., Depierre J. W., Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979 Jan 4;582(1):67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- Ono K., Shrieve D. C. Enhancement of EMT6/SF tumor cell killing by mitomycin C and cyclophosphamide following in vivo administration of buthionine sulfoximine. Int J Radiat Oncol Biol Phys. 1986 Jul;12(7):1175–1178. doi: 10.1016/0360-3016(86)90252-x. [DOI] [PubMed] [Google Scholar]
- Russo A., Tochner Z., Phillips T., Carmichael J., DeGraff W., Friedman N., Fisher J., Mitchell J. B. In vivo modulation of glutathione by buthionine sulfoximine: effect on marrow response to melphalan. Int J Radiat Oncol Biol Phys. 1986 Jul;12(7):1187–1189. doi: 10.1016/0360-3016(86)90255-5. [DOI] [PubMed] [Google Scholar]


