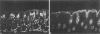Abstract
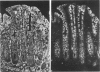
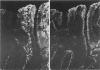
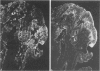
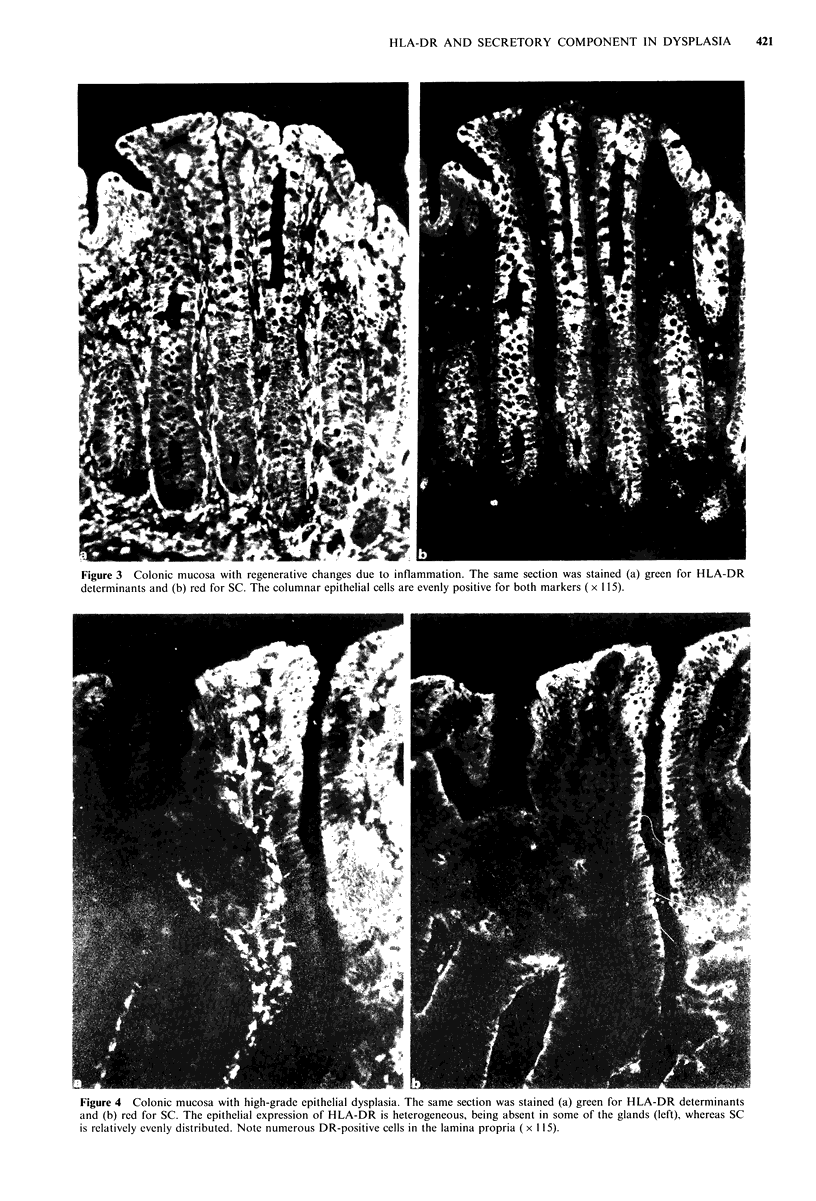
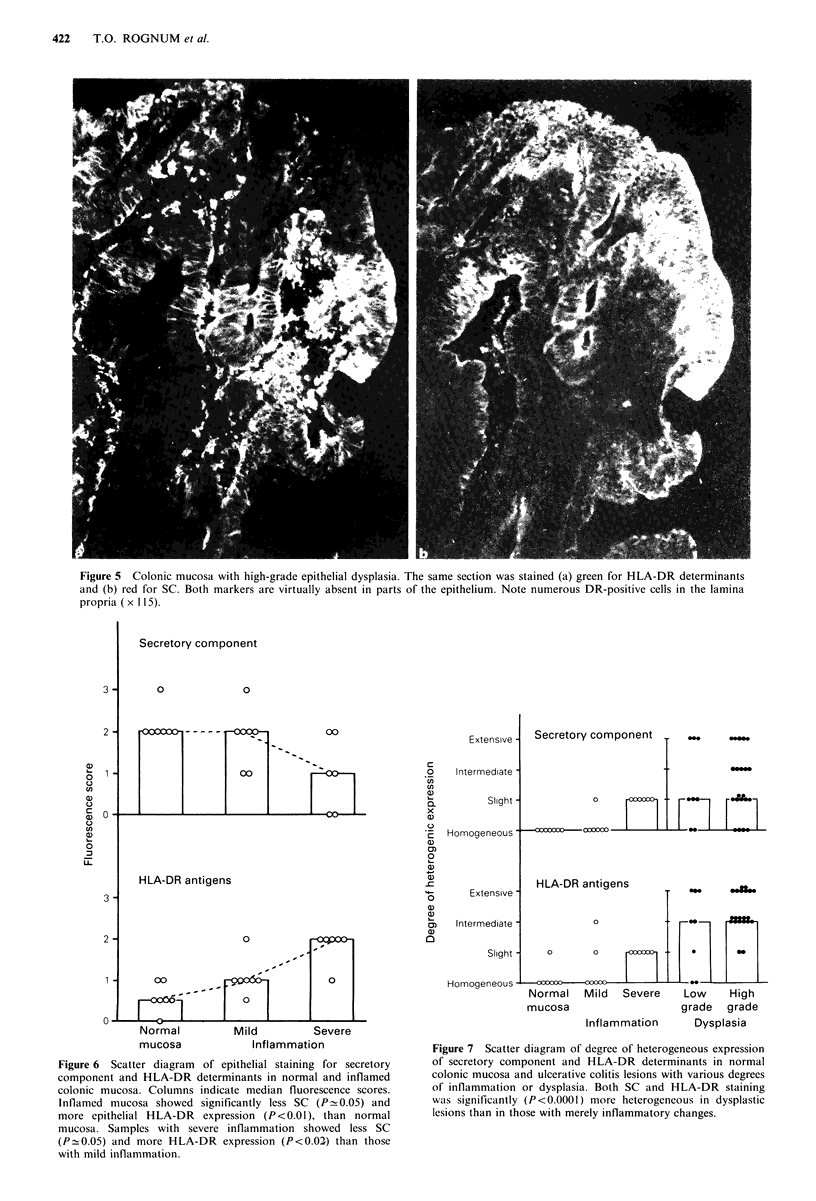
The intensity and degree of heterogeneous epithelial marker expression were evaluated immunohistochemically in 29 mucosal biopsy specimens from 7 ulcerative colitis (UC) patients with dysplasia. Biopsy specimens from UC patients with mild (n = 7) or severe (n = 6) inflammation and from histologically normal samples (n = 7) served as controls. HLA-DR showed heterogeneous epithelial expression in all lesions with high grade dysplasia and in 6 of 8 with low grade dysplasia. SC was heterogeneous stained in 17 of 21 lesions with high grade dysplasia and in all but two lesions with low grade dysplasia. In histologically normal mucosa, SC was homogeneously expressed and epithelial DR was virtually absent. In mildly inflamed UC lesions, SC exhibited patchy distribution in only one sample and DR in two, whereas both SC and DR showed a slight degree of heterogeneous expression in all lesions with severe inflammation. Moreover, the overall intensity of SC staining tended to decrease with increasing degree of inflammation, whereas the opposite was seen for DR. Decreased SC and increased DR expression thus seemed to be related to intensified inflammatory activity, whereas heterogeneous expression of these markers was significantly more related to dysplasia.
Full text
PDF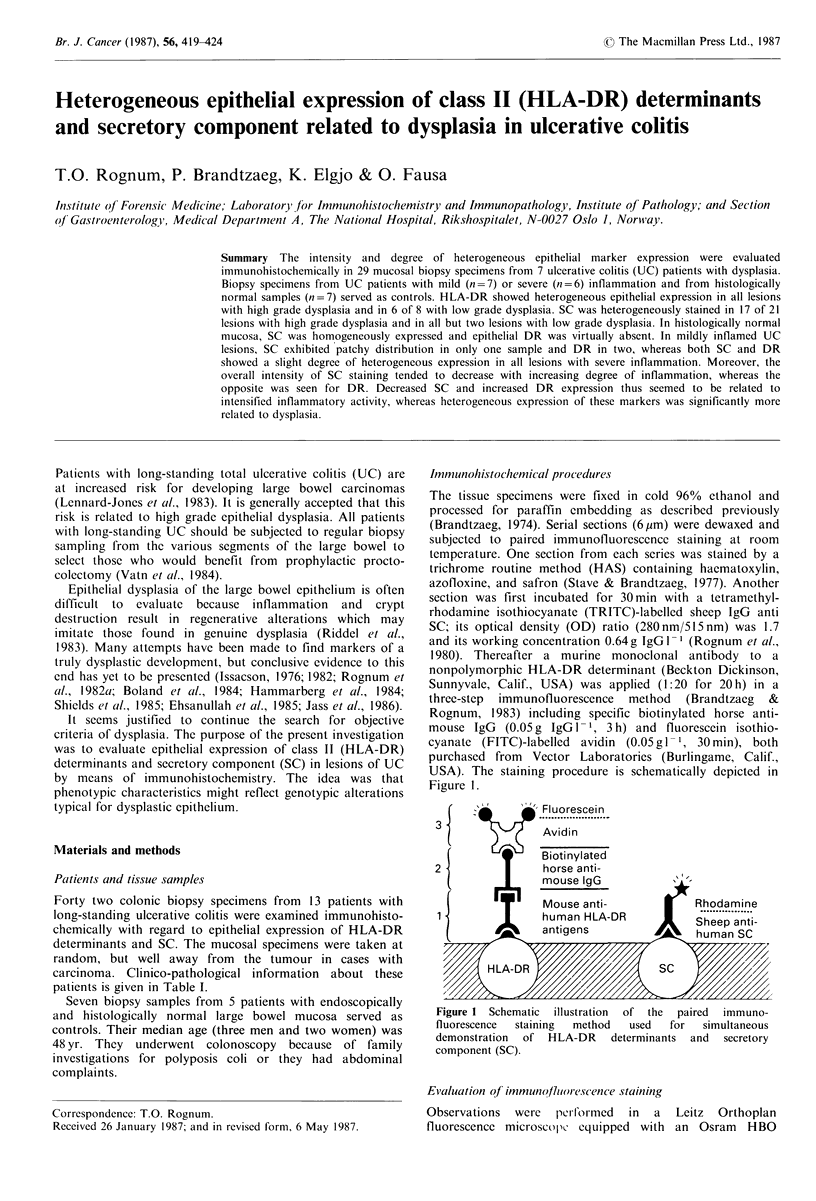



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boland C. R., Lance P., Levin B., Riddell R. H., Kim Y. S. Abnormal goblet cell glycoconjugates in rectal biopsies associated with an increased risk of neoplasia in patients with ulcerative colitis: early results of a prospective study. Gut. 1984 Dec;25(12):1364–1371. doi: 10.1136/gut.25.12.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P., Rognum T. O. Evaluation of tissue preparation methods and paired immunofluorescence staining for immunocytochemistry of lymphomas. Histochem J. 1983 Jul;15(7):655–689. doi: 10.1007/BF01002987. [DOI] [PubMed] [Google Scholar]
- Daar A. S., Fabre J. W. The membrane antigens of human colorectal cancer cells: demonstration with monoclonal antibodies of heterogeneity within and between tumours and of anomalous expression of HLA-DR. Eur J Cancer Clin Oncol. 1983 Feb;19(2):209–220. doi: 10.1016/0277-5379(83)90419-4. [DOI] [PubMed] [Google Scholar]
- Daar A. S., Fuggle S. V., Ting A., Fabre J. W. Anomolous expression of HLA-DR antigens on human colorectal cancer cells. J Immunol. 1982 Aug;129(2):447–449. [PubMed] [Google Scholar]
- Ehsanullah M., Naunton Morgan M., Filipe M. I., Gazzard B. Sialomucins in the assessment of dysplasia and cancer-risk patients with ulcerative colitis treated with colectomy and ileo-rectal anastomosis. Histopathology. 1985 Feb;9(2):223–235. doi: 10.1111/j.1365-2559.1985.tb02437.x. [DOI] [PubMed] [Google Scholar]
- Ghosh A. K., Moore M., Street A. J., Howat J. M., Schofield P. F. Expression of HLA-D sub-region products on human colorectal carcinoma. Int J Cancer. 1986 Oct 15;38(4):459–464. doi: 10.1002/ijc.2910380402. [DOI] [PubMed] [Google Scholar]
- Hamilton S. R., Keren D. F., Boitnott J. K., Robertson S. M., Yardley J. H. Enhancement by cholera toxin of IgA secretion from intestinal crypt epithelium. Gut. 1980 May;21(5):365–369. doi: 10.1136/gut.21.5.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarberg C., Slezak P., Tribukait B. Early detection of malignancy in ulcerative colitis. A flow-cytometric DNA study. Cancer. 1984 Jan 15;53(2):291–295. doi: 10.1002/1097-0142(19840115)53:2<291::aid-cncr2820530218>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Isaacson P. Immunoperoxidase study of the secretory immunoglobulin system in colonic neoplasia. J Clin Pathol. 1982 Jan;35(1):14–25. doi: 10.1136/jcp.35.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson P. Tissue demonstration of carcinoembryonic antigen (CEA) in ulcerative colitis. Gut. 1976 Jul;17(7):561–567. doi: 10.1136/gut.17.7.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jass J. R., England J., Miller K. Value of mucin histochemistry in follow up surveillance of patients with long standing ulcerative colitis. J Clin Pathol. 1986 Apr;39(4):393–398. doi: 10.1136/jcp.39.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald G. B., Jewell D. P. Class II antigen (HLA-DR) expression by intestinal epithelial cells in inflammatory diseases of colon. J Clin Pathol. 1987 Mar;40(3):312–317. doi: 10.1136/jcp.40.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M., Ghosh A. K., Johnston D., Street A. J. Expression of MHC class II products on human colorectal cancer. An immunohistological and flow cytometric study. J Immunogenet. 1986 Apr-Jun;13(2-3):201–209. doi: 10.1111/j.1744-313x.1986.tb01102.x. [DOI] [PubMed] [Google Scholar]
- Nowell P. C. Mechanisms of tumor progression. Cancer Res. 1986 May;46(5):2203–2207. [PubMed] [Google Scholar]
- Nowell P. C. The clonal evolution of tumor cell populations. Science. 1976 Oct 1;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Cotran R. S., Reiss C. S., Burakoff S. J., Fiers W., Ault K. A. Ia expression by vascular endothelium is inducible by activated T cells and by human gamma interferon. J Exp Med. 1983 Apr 1;157(4):1339–1353. doi: 10.1084/jem.157.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen L. O., Elling P., Sørensen F. B., Høedt-Rasmussen K. HLA-DR expression and disease activity in ulcerative colitis. Scand J Gastroenterol. 1986 Apr;21(3):364–368. doi: 10.3109/00365528609003088. [DOI] [PubMed] [Google Scholar]
- Riddell R. H., Goldman H., Ransohoff D. F., Appelman H. D., Fenoglio C. M., Haggitt R. C., Ahren C., Correa P., Hamilton S. R., Morson B. C. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983 Nov;14(11):931–968. doi: 10.1016/s0046-8177(83)80175-0. [DOI] [PubMed] [Google Scholar]
- Rognum T. O., Brandtzaeg P., Baklien K., Hognestad J. Immunoglobulin-producing cells in the "transitional" mucosa adjacent to adenocarcinomas of the human large bowel. Int J Cancer. 1979 Feb;23(2):165–173. doi: 10.1002/ijc.2910230205. [DOI] [PubMed] [Google Scholar]
- Rognum T. O., Brandtzaeg P., Thorud E. Is heterogeneous expression of HLA-dr antigens and CEA along with DNA-profile variations evidence of phenotypic instability and clonal proliferation in human large bowel carcinomas? Br J Cancer. 1983 Oct;48(4):543–551. doi: 10.1038/bjc.1983.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognum T. O., Elgjo K., Fausa O., Brandtzaeg P. Immunohistochemical evaluation of carcinoembryonic antigen, secretory component, and epithelial IgA in ulcerative colitis with dysplasia. Gut. 1982 Feb;23(2):123–133. doi: 10.1136/gut.23.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognum T. O., Fausa O., Brandtzaeg P. Immunohistochemical evaluation of carcinoembryonic antigen, secretory component, and epithelial IgA in tubular and villous large-bowel adenomas with different grades of dysplasia. Scand J Gastroenterol. 1982 Apr;17(3):341–348. doi: 10.3109/00365528209182065. [DOI] [PubMed] [Google Scholar]
- Selby W. S., Janossy G., Mason D. Y., Jewell D. P. Expression of HLA-DR antigens by colonic epithelium in inflammatory bowel disease. Clin Exp Immunol. 1983 Sep;53(3):614–618. [PMC free article] [PubMed] [Google Scholar]
- Sollid L. M., Kvale D., Brandtzaeg P., Markussen G., Thorsby E. Interferon-gamma enhances expression of secretory component, the epithelial receptor for polymeric immunoglobulins. J Immunol. 1987 Jun 15;138(12):4303–4306. [PubMed] [Google Scholar]
- Stave R., Brandtzaeg P. Fluorescence staining of gastric mucosa. A study with special reference to parietal cells. Scand J Gastroenterol. 1977;12(7):885–891. doi: 10.3109/00365527709181735. [DOI] [PubMed] [Google Scholar]
- Svennevig J. L. In situ identification of inflammatory cells in malignant, non-lymphoid human tumours. Acta Pathol Microbiol Scand A. 1980 Nov;88(6):387–395. doi: 10.1111/j.1699-0463.1980.tb02511.x. [DOI] [PubMed] [Google Scholar]
- Valnes K., Brandtzaeg P., Elgjo K., Stave R. Specific and nonspecific humoral defense factors in the epithelium of normal and inflamed gastric mucosa. Immunohistochemical localization of immunoglobulins, secretory component, lysozyme, and lactoferrin. Gastroenterology. 1984 Mar;86(3):402–412. [PubMed] [Google Scholar]
- Vatn M. H., Elgjo K., Bergan A. Distribution of dysplasia in ulcerative colitis. Scand J Gastroenterol. 1984 Oct;19(7):893–895. [PubMed] [Google Scholar]
- Yardley J. H., Keren D. F. "Precancer" lesions in ulcerative colits. A retrospective study of rectal biopsy and colectomy specimens. Cancer. 1974 Sep;34(3):suppl–suppl:844. doi: 10.1002/1097-0142(197409)34:3+<835::aid-cncr2820340709>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]