Abstract
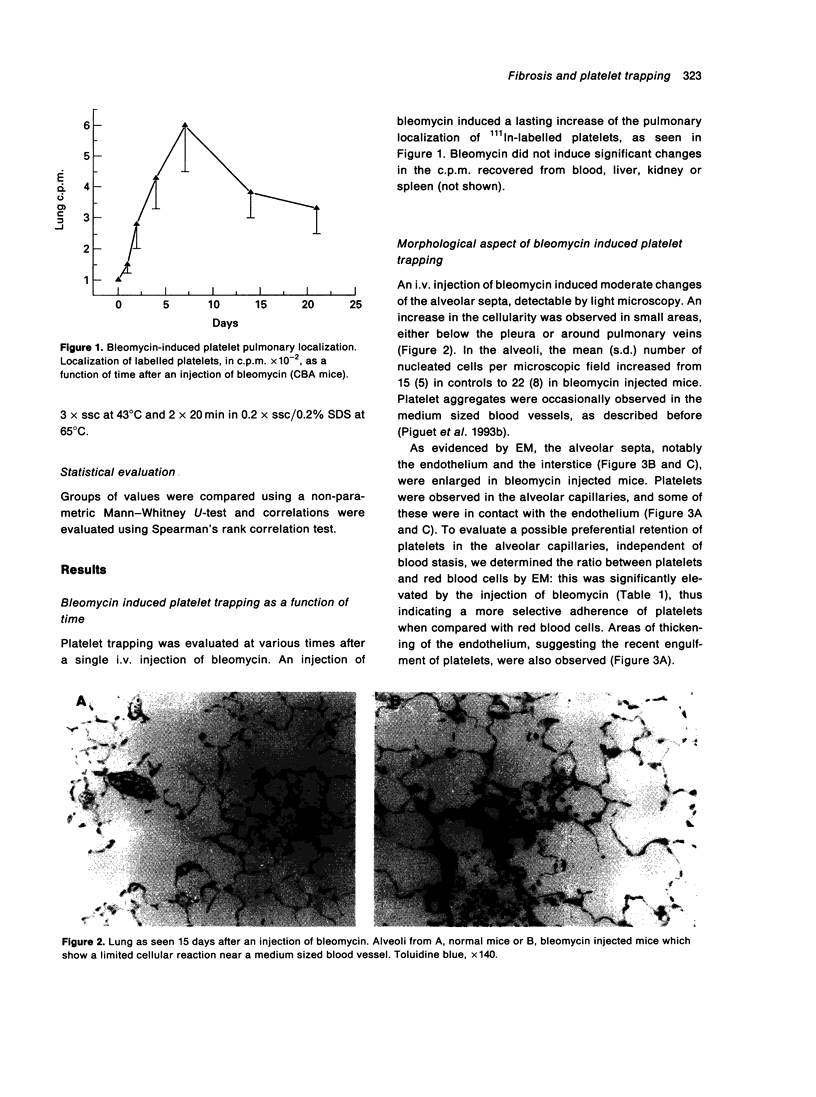
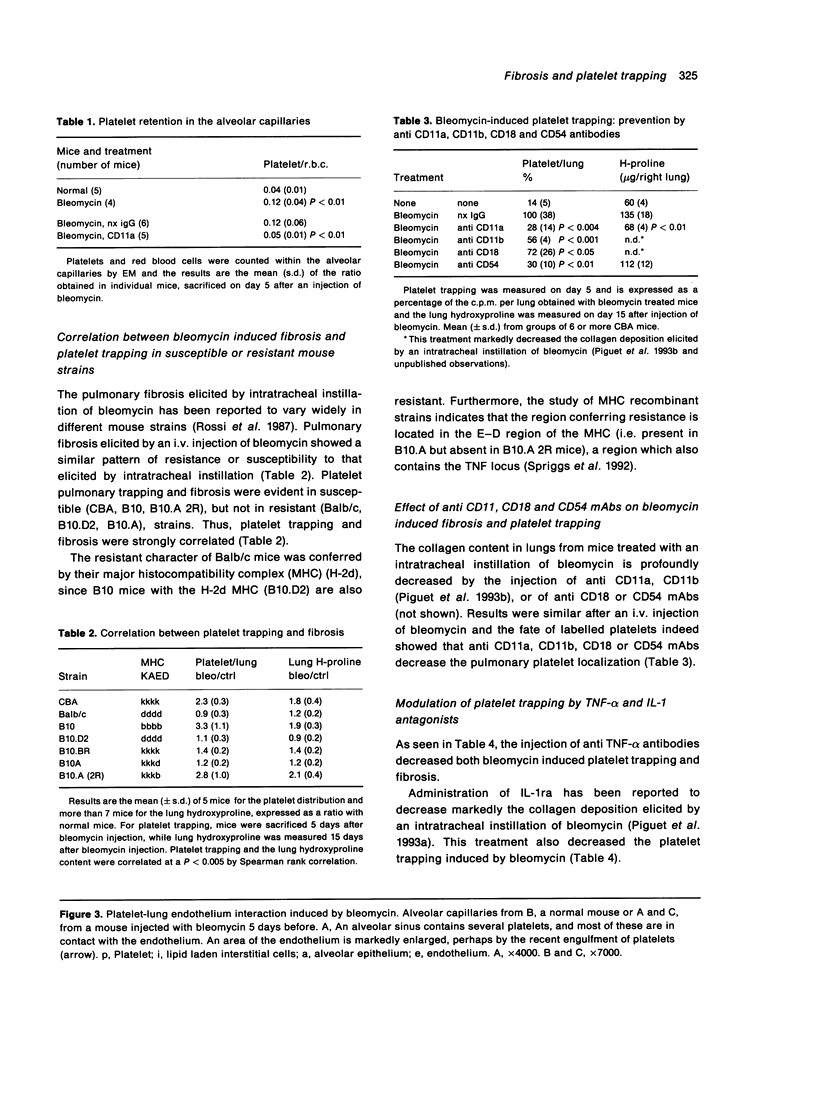
Platelet trapping was explored during the course of bleomycin induced pulmonary fibrosis by the injection of indium-111 labelled platelets and by light and electron microscopy (EM) of the alveolar capillaries. An i.v. injection of bleomycin markedly increased the localization of labelled platelets in the lung (but not in other organs) for about 3 weeks. On day 7 after bleomycin injection, a significant increase in the number of platelets in contact with the alveolar endothelium was seen with EM. Platelet trapping was strongly correlated (P < 0.005) with collagen deposition when examined in mouse strains genetically susceptible (CBA, C57BL/10, BL10 A.2R), or resistant (Balb/c, BL10.D2, BL10.A), to bleomycin induced fibrosis. In addition, several treatments known to decrease bleomycin induced collagen deposition and synthesis, namely administration of antibodies against CD11a, CD11b, TNF-alpha and IL-1ra, also decreased platelet trapping. As evaluated by EM, anti CD11a mAb significantly decreased the number of platelets in contact with the alveolar endothelium. This study indicates that bleomycin induced pulmonary fibrosis is strongly correlated with platelet trapping and that platelets probably interact, via their CD11a, with the CD54 born by the alveolar endothelium.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assoian R. K., Sporn M. B. Type beta transforming growth factor in human platelets: release during platelet degranulation and action on vascular smooth muscle cells. J Cell Biol. 1986 Apr;102(4):1217–1223. doi: 10.1083/jcb.102.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T. W., McAnulty R. J., Rogers D. F., Chung K. F., Barnes P. J., Laurent G. J. Bleomycin-induced lung injury in the rat: effects of the platelet-activating factor (PAF) receptor antagonist BN 52021 and platelet depletion. Environ Health Perspect. 1990 Apr;85:65–69. doi: 10.1289/ehp.85-1568314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese C., Rowe D., Kream B. Construction of DNA sequences complementary to rat alpha 1 and alpha 2 collagen mRNA and their use in studying the regulation of type I collagen synthesis by 1,25-dihydroxyvitamin D. Biochemistry. 1984 Dec 4;23(25):6210–6216. doi: 10.1021/bi00320a049. [DOI] [PubMed] [Google Scholar]
- Grau G. E., Tacchini-Cottier F., Vesin C., Milon G., Lou J. N., Piguet P. F., Juillard P. TNF-induced microvascular pathology: active role for platelets and importance of the LFA-1/ICAM-1 interaction. Eur Cytokine Netw. 1993 Nov-Dec;4(6):415–419. [PubMed] [Google Scholar]
- Huszar G., Maiocco J., Naftolin F. Monitoring of collagen and collagen fragments in chromatography of protein mixtures. Anal Biochem. 1980 Jul 1;105(2):424–429. doi: 10.1016/0003-2697(80)90481-9. [DOI] [PubMed] [Google Scholar]
- Kovacs E. J. Fibrogenic cytokines: the role of immune mediators in the development of scar tissue. Immunol Today. 1991 Jan;12(1):17–23. doi: 10.1016/0167-5699(91)90107-5. [DOI] [PubMed] [Google Scholar]
- Kunicki T. J., Newman P. J. The molecular immunology of human platelet proteins. Blood. 1992 Sep 15;80(6):1386–1404. [PubMed] [Google Scholar]
- McCaffery P. J., Berridge M. V. Expression of the leukocyte functional molecule (LFA-1) on mouse platelets. Blood. 1986 Jun;67(6):1757–1764. [PubMed] [Google Scholar]
- Phan S. H., Kunkel S. L. Lung cytokine production in bleomycin-induced pulmonary fibrosis. Exp Lung Res. 1992 Jan-Mar;18(1):29–43. doi: 10.3109/01902149209020649. [DOI] [PubMed] [Google Scholar]
- Pierres M., Goridis C., Golstein P. Inhibition of murine T cell-mediated cytolysis and T cell proliferation by a rat monoclonal antibody immunoprecipitating two lymphoid cell surface polypeptides of 94 000 and 180 000 molecular weight. Eur J Immunol. 1982 Jan;12(1):60–69. doi: 10.1002/eji.1830120112. [DOI] [PubMed] [Google Scholar]
- Piguet P. F., Collart M. A., Grau G. E., Kapanci Y., Vassalli P. Tumor necrosis factor/cachectin plays a key role in bleomycin-induced pneumopathy and fibrosis. J Exp Med. 1989 Sep 1;170(3):655–663. doi: 10.1084/jem.170.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet P. F., Rosen H., Vesin C., Grau G. E. Effective treatment of the pulmonary fibrosis elicited in mice by bleomycin or silica with anti-CD-11 antibodies. Am Rev Respir Dis. 1993 Feb;147(2):435–441. doi: 10.1164/ajrccm/147.2.435. [DOI] [PubMed] [Google Scholar]
- Piguet P. F., Vesin C., Grau G. E., Thompson R. C. Interleukin 1 receptor antagonist (IL-1ra) prevents or cures pulmonary fibrosis elicited in mice by bleomycin or silica. Cytokine. 1993 Jan;5(1):57–61. doi: 10.1016/1043-4666(93)90024-y. [DOI] [PubMed] [Google Scholar]
- Piguet P. F., Vesin C., Ryser J. E., Senaldi G., Grau G. E., Tacchini-Cottier F. An effector role for platelets in systemic and local lipopolysaccharide-induced toxicity in mice, mediated by a CD11a- and CD54-dependent interaction with endothelium. Infect Immun. 1993 Oct;61(10):4182–4187. doi: 10.1128/iai.61.10.4182-4187.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto J., Takei F., Gendelman R., Christenson B., Biberfeld P., Patarroyo M. MALA-2, mouse homologue of human adhesion molecule ICAM-1 (CD54). Eur J Immunol. 1989 Sep;19(9):1551–1557. doi: 10.1002/eji.1830190906. [DOI] [PubMed] [Google Scholar]
- Rosen H., Gordon S. Monoclonal antibody to the murine type 3 complement receptor inhibits adhesion of myelomonocytic cells in vitro and inflammatory cell recruitment in vivo. J Exp Med. 1987 Dec 1;166(6):1685–1701. doi: 10.1084/jem.166.6.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum W. I. Aspects of endothelial malfunction and function in cerebral microvessels. Lab Invest. 1986 Sep;55(3):252–268. [PubMed] [Google Scholar]
- Ross R., Glomset J., Kariya B., Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi G. A., Szapiel S., Ferrans V. J., Crystal R. G. Susceptibility to experimental interstitial lung disease is modified by immune- and non-immune-related genes. Am Rev Respir Dis. 1987 Feb;135(2):448–455. doi: 10.1164/arrd.1987.135.2.448. [DOI] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Simon P., Thompson S., Springer T. A. Mapping of antigenic and functional epitopes on the alpha- and beta-subunits of two related mouse glycoproteins involved in cell interactions, LFA-1 and Mac-1. J Exp Med. 1983 Aug 1;158(2):586–602. doi: 10.1084/jem.158.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrall R. S., McCormick J. R., Jack R. M., McReynolds R. A., Ward P. A. Bleomycin-induced pulmonary fibrosis in the rat: inhibition by indomethacin. Am J Pathol. 1979 Apr;95(1):117–130. [PMC free article] [PubMed] [Google Scholar]
- Wojcik J. D., Van Horn D. L., Webber A. J., Johnson S. A. Mechanism whereby platelets support the endothelium. Transfusion. 1969 Nov-Dec;9(6):324–335. doi: 10.1111/j.1537-2995.1969.tb04945.x. [DOI] [PubMed] [Google Scholar]




