Abstract
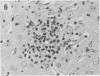
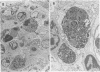
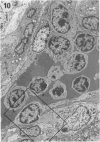
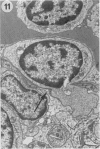
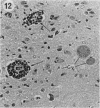
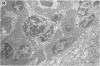
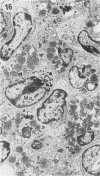
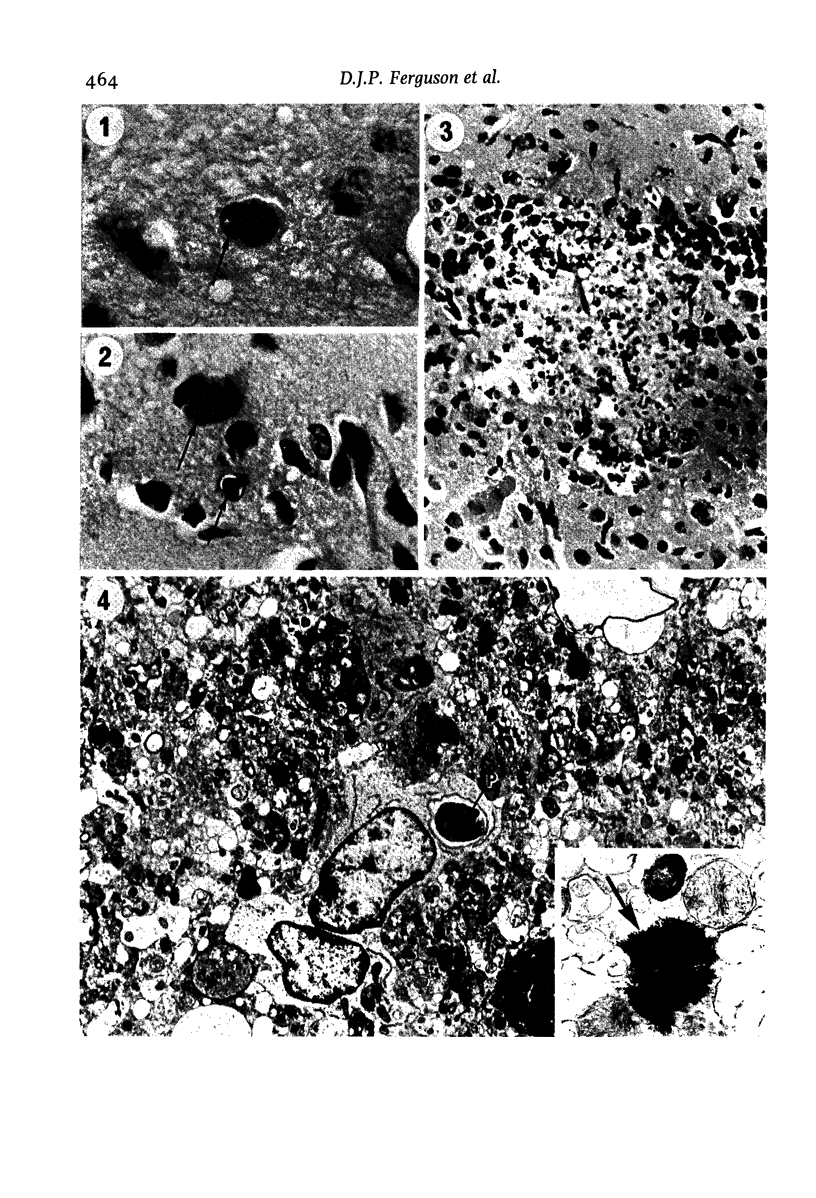
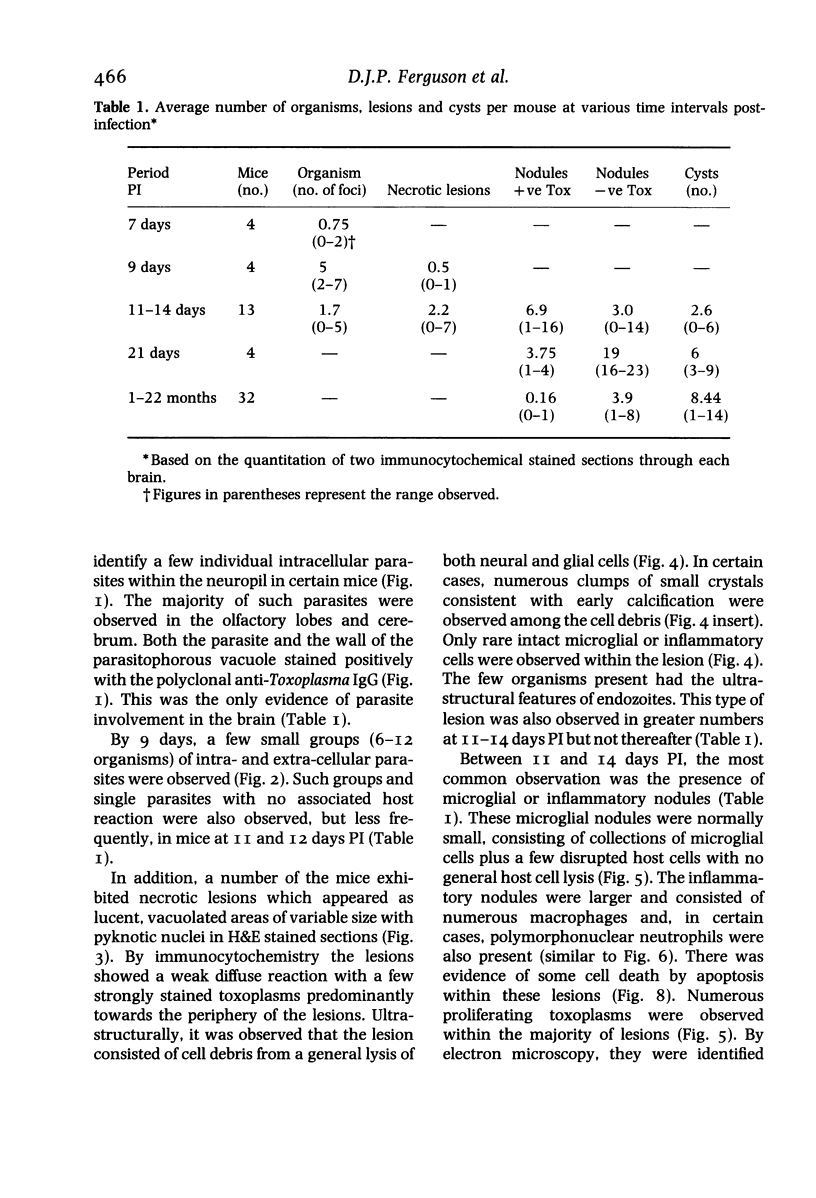
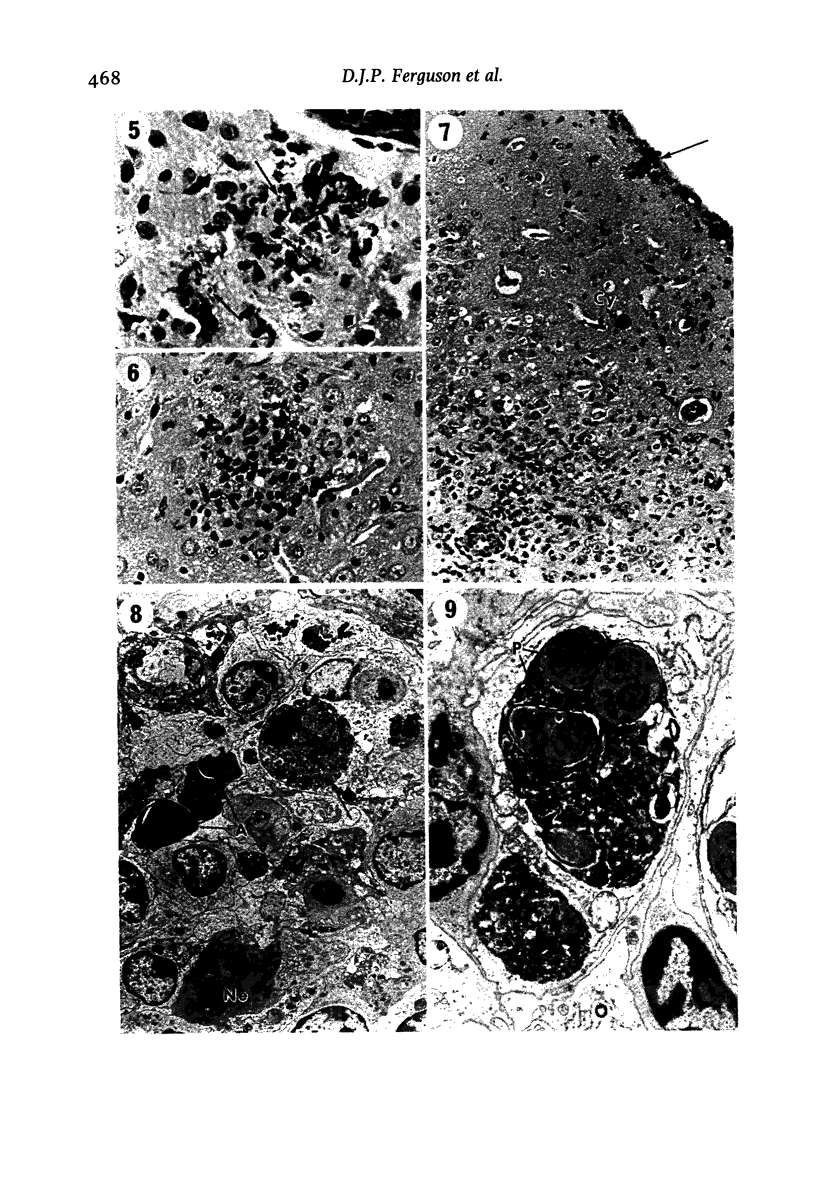
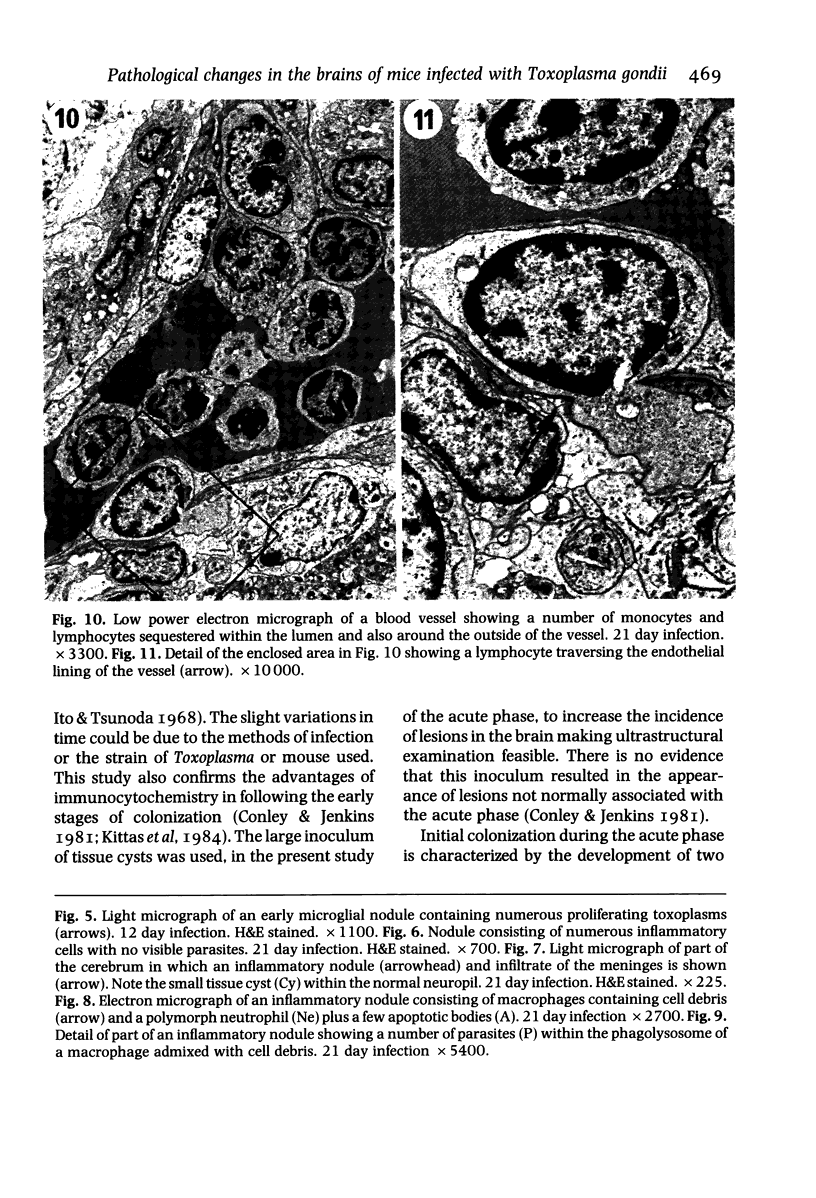
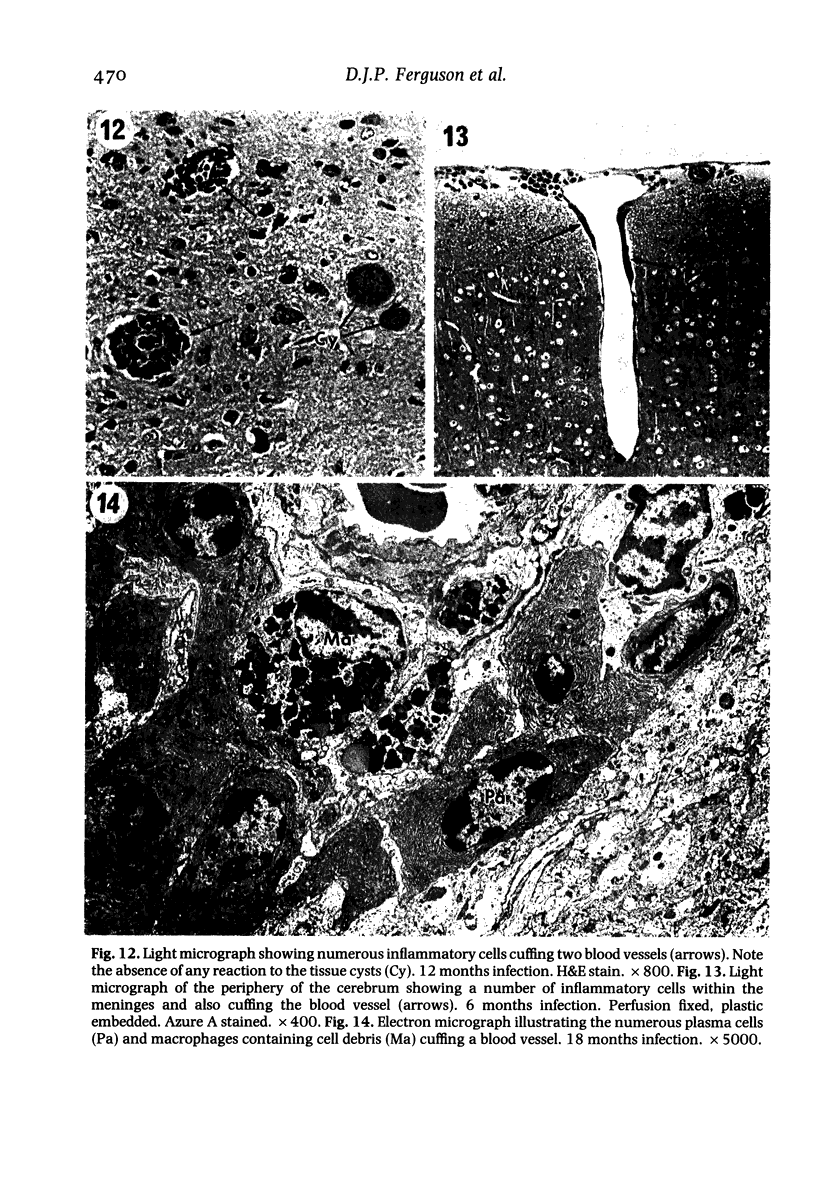
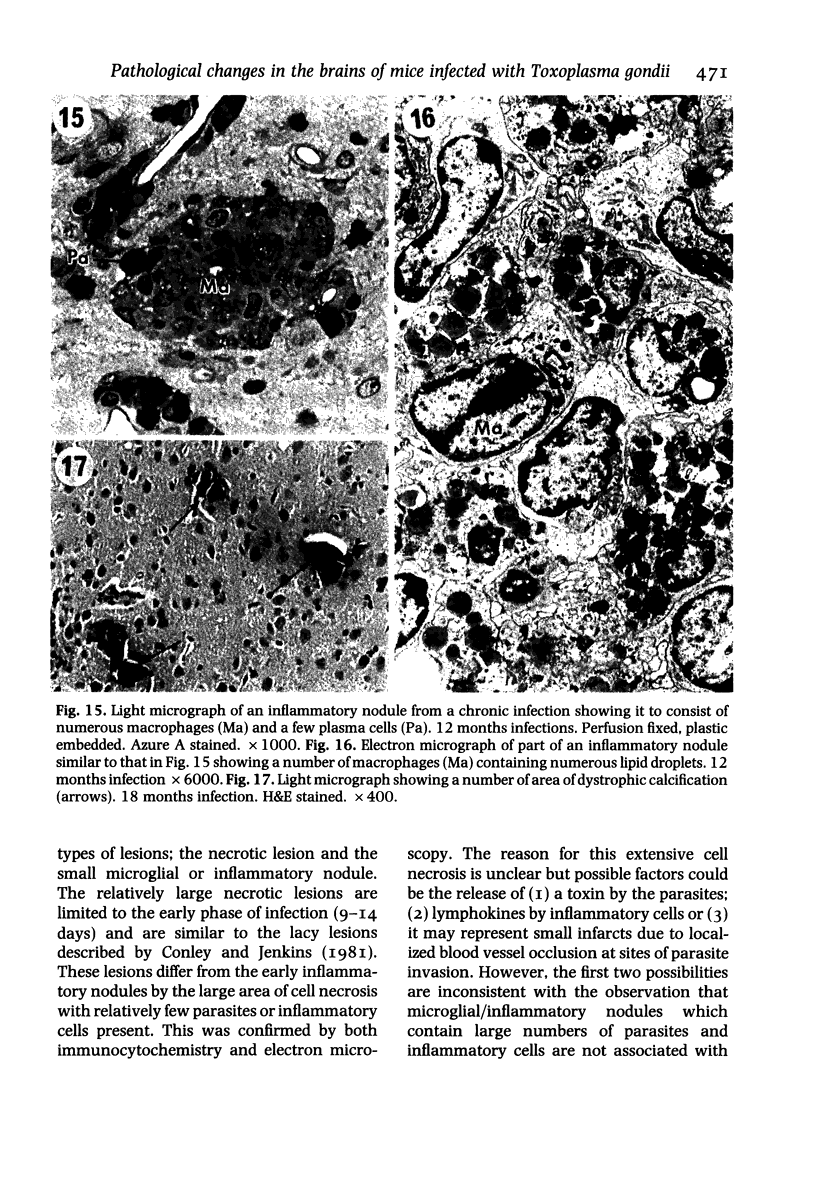
The pathological changes in the brains of mice infected with T. gondii were studied at various intervals between 7 days and 22 months post-infection using histology, immunocytochemistry and electron microscopy. Initially, a few single parasites were observed (day 7) but necrotic lesions and microglial and inflammatory nodules rapidly appeared (9-I4 days). The majority of the lesions between days 9 and I4 contained proliferating toxoplasma and early cyst formation but from 2I days onwards the vast majority of nodules contained neither parasites nor Toxoplasma antigen. Intact intracellular cysts persisted throughout the period of study eliciting no host response. A generalized meningoencephalitis developed by day II and persisted with varying degrees of severity throughout the 22 months studied. At first, the inflammatory cells consisted of lymphocytes and monocyte/macrophages but during the chronic phase plasma cells predominated. In chronic infections, the number of microglial/inflammatory nodules was relatively constant with only a few containing toxoplasmic material resulting from recent cyst rupture. A few brains contained small nodules of dystrophic calcification. This study shows that in these asymptomatic animals, the major feature is perivascular cuffing by mononuclear cells and localized microglial/inflammatory nodules. After the development of the chronic state, there is no obvious increase or decrease in the severity of the pathological changes with time.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carson F. L., Martin J. H., Lynn J. A. Formalin fixation for electron microscopy: a re-evaluation. Am J Clin Pathol. 1973 Mar;59(3):365–373. doi: 10.1093/ajcp/59.3.365. [DOI] [PubMed] [Google Scholar]
- Ferguson D. J., Hutchison W. M. An ultrastructural study of the early development and tissue cyst formation of Toxoplasma gondii in the brains of mice. Parasitol Res. 1987;73(6):483–491. doi: 10.1007/BF00535321. [DOI] [PubMed] [Google Scholar]
- Frenkel J. K., Escajadillo A. Cyst rupture as a pathogenic mechanism of toxoplasmic encephalitis. Am J Trop Med Hyg. 1987 May;36(3):517–522. doi: 10.4269/ajtmh.1987.36.517. [DOI] [PubMed] [Google Scholar]
- Frenkel J. K., Nelson B. M., Arias-Stella J. Immunosuppression and toxoplasmic encephalitis: clinical and experimental aspects. Hum Pathol. 1975 Jan;6(1):97–111. doi: 10.1016/s0046-8177(75)80111-0. [DOI] [PubMed] [Google Scholar]
- Graham D. I., Hay J., Hutchison W. M., Siim J. C. Encephalitis in mice with congenital ocular toxoplasmosis. J Pathol. 1984 Apr;142(4):265–277. doi: 10.1002/path.1711420405. [DOI] [PubMed] [Google Scholar]
- Hutchison W. M. Strain of mouse and Toxoplasma used in the mouse model of congenital toxoplasmosis at Strathclyde University. Ann Trop Med Parasitol. 1986 Apr;80(2):253–255. doi: 10.1080/00034983.1986.11812010. [DOI] [PubMed] [Google Scholar]
- Jacobs L. Toxoplasma and toxoplasmosis. Adv Parasitol. 1967;5:1–45. doi: 10.1016/s0065-308x(08)60374-7. [DOI] [PubMed] [Google Scholar]
- Kittas S., Kittas C., Paizi-Biza P., Henry L. A histological and immunohistochemical study of the changes induced in the brains of white mice by infection with Toxoplasma gondii. Br J Exp Pathol. 1984 Feb;65(1):67–74. [PMC free article] [PubMed] [Google Scholar]
- Navia B. A., Petito C. K., Gold J. W., Cho E. S., Jordan B. D., Price R. W. Cerebral toxoplasmosis complicating the acquired immune deficiency syndrome: clinical and neuropathological findings in 27 patients. Ann Neurol. 1986 Mar;19(3):224–238. doi: 10.1002/ana.410190303. [DOI] [PubMed] [Google Scholar]
- Raine C. S., Cannella B., Duijvestijn A. M., Cross A. H. Homing to central nervous system vasculature by antigen-specific lymphocytes. II. Lymphocyte/endothelial cell adhesion during the initial stages of autoimmune demyelination. Lab Invest. 1990 Oct;63(4):476–489. [PubMed] [Google Scholar]
- Remington J. S., Krahenbuhl J. L., Mendenhall J. W. A role for activated macrophages in resistance to infection with Toxoplasma. Infect Immun. 1972 Nov;6(5):829–834. doi: 10.1128/iai.6.5.829-834.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoolman L. M. Adhesion molecules controlling lymphocyte migration. Cell. 1989 Mar 24;56(6):907–910. doi: 10.1016/0092-8674(89)90620-x. [DOI] [PubMed] [Google Scholar]
- Tadros W., Laarman J. J. Current concepts on the biology, evolution and taxonomy of tissue cyst-forming eimeriid coccidia. Adv Parasitol. 1982;20:293–468. doi: 10.1016/s0065-308x(08)60540-0. [DOI] [PubMed] [Google Scholar]