Abstract
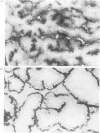
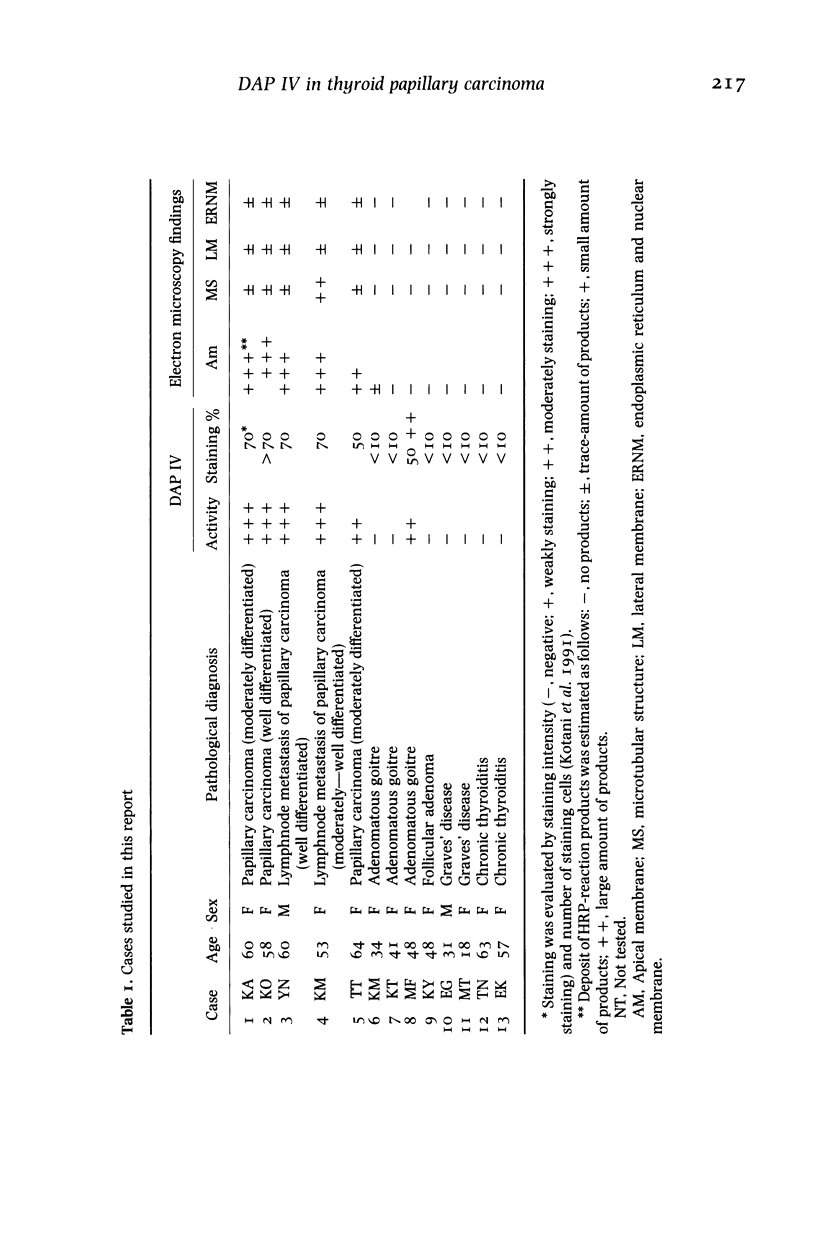
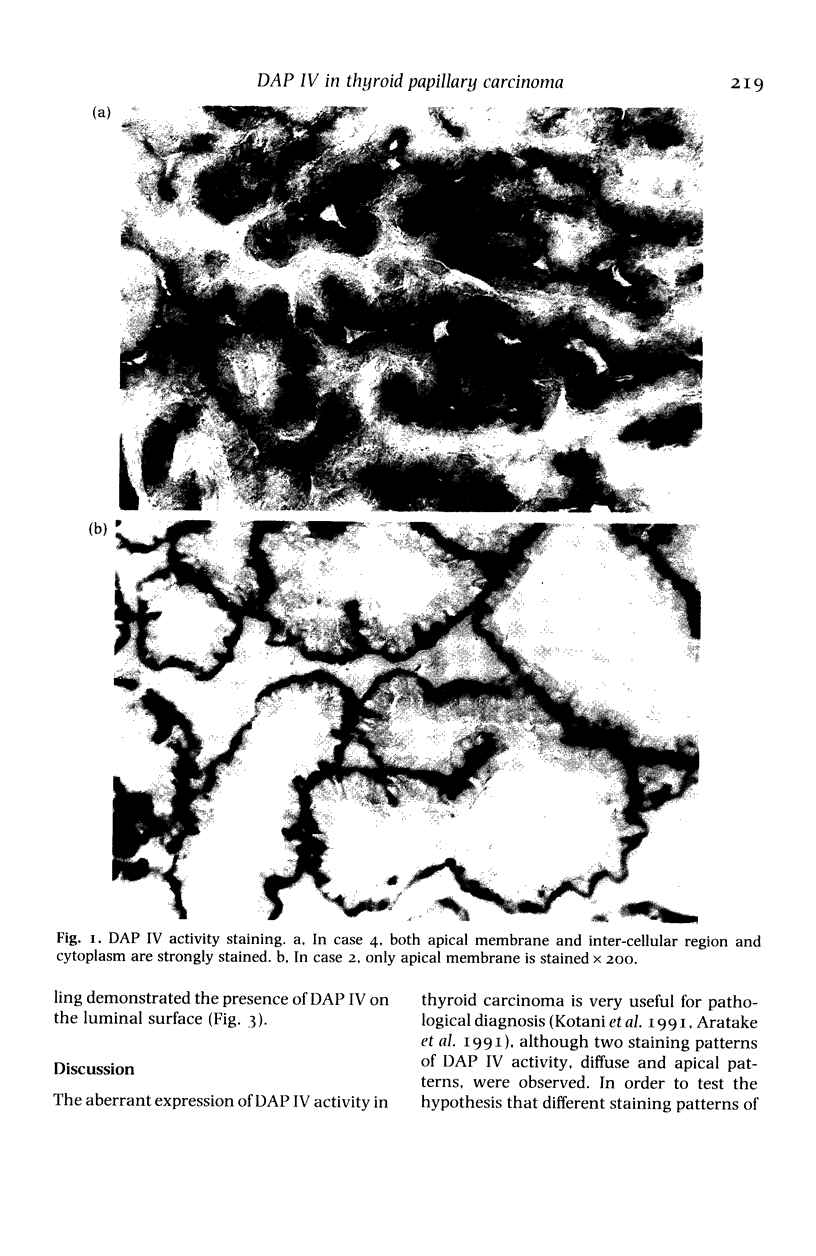
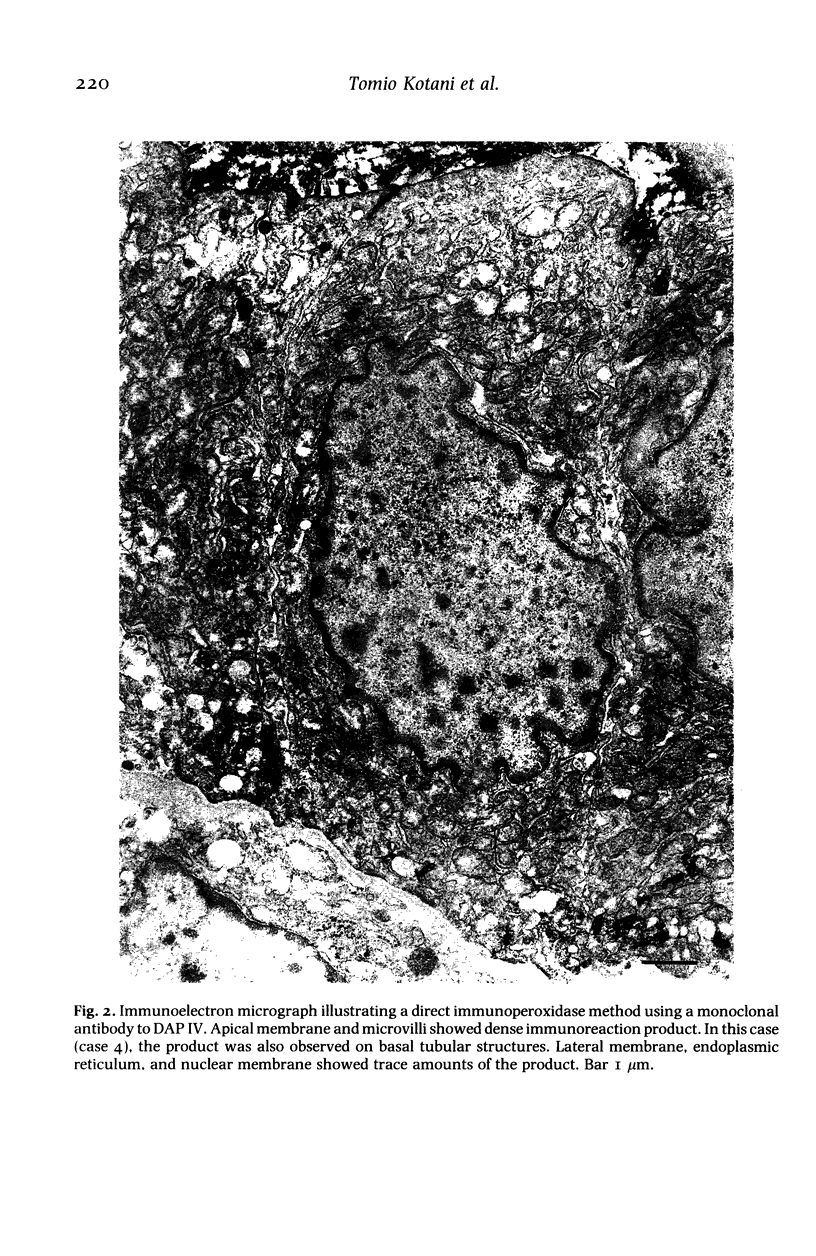
The localization of dipeptidyl aminopeptidase IV expressed aberrantly in thyroid carcinoma was studied by immunoelectron microscopy using a monoclonal antibody to the enzyme with special reference to enzyme-histochemical staining of the enzyme. Five thyroid papillary carcinomas were investigated including two lymph-node metastases. All cases showed the dense immunoreaction product on the apical membrane and only traces of the product on lateral membranes, endoplasmic reticulum and nuclear membranes. In one case only, the dense product was observed on basal tubular structures. Analysis, using immunogold labelling on pre-embedded cryosections, revealed that dipeptidyl aminopeptidase IV was localized on the luminal surface of cancer cells. Two different distribution patterns of dipeptidyl aminopeptidase IV activity staining, diffuse and apical patterns, reported previously were thought to be due to different amounts of dipeptidyl aminopeptidase IV in the cytoplasm of cancer cells. This enzyme-histochemical staining method is useful for pathological diagnosis of thyroid tumours and can be applied to clinical materials. The enzyme localization is revealed by the staining pattern.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aratake Y., Kotani T., Tamura K., Araki Y., Kuribayashi T., Konoe K., Ohtaki S. Dipeptidyl aminopeptidase IV staining of cytologic preparations to distinguish benign from malignant thyroid diseases. Am J Clin Pathol. 1991 Sep;96(3):306–310. doi: 10.1093/ajcp/96.3.306. [DOI] [PubMed] [Google Scholar]
- Hopsu-Havu V. K., Glenner G. G. A new dipeptide naphthylamidase hydrolyzing glycyl-prolyl-beta-naphthylamide. Histochemie. 1966;7(3):197–201. doi: 10.1007/BF00577838. [DOI] [PubMed] [Google Scholar]
- Nagatsu T., Hino M., Fuyamada H., Hayakawa T., Sakakibara S. New chromogenic substrates for X-prolyl dipeptidyl-aminopeptidase. Anal Biochem. 1976 Aug;74(2):466–476. doi: 10.1016/0003-2697(76)90227-x. [DOI] [PubMed] [Google Scholar]
- Ogata S., Misumi Y., Ikehara Y. Primary structure of rat liver dipeptidyl peptidase IV deduced from its cDNA and identification of the NH2-terminal signal sequence as the membrane-anchoring domain. J Biol Chem. 1989 Feb 25;264(6):3596–3601. [PubMed] [Google Scholar]