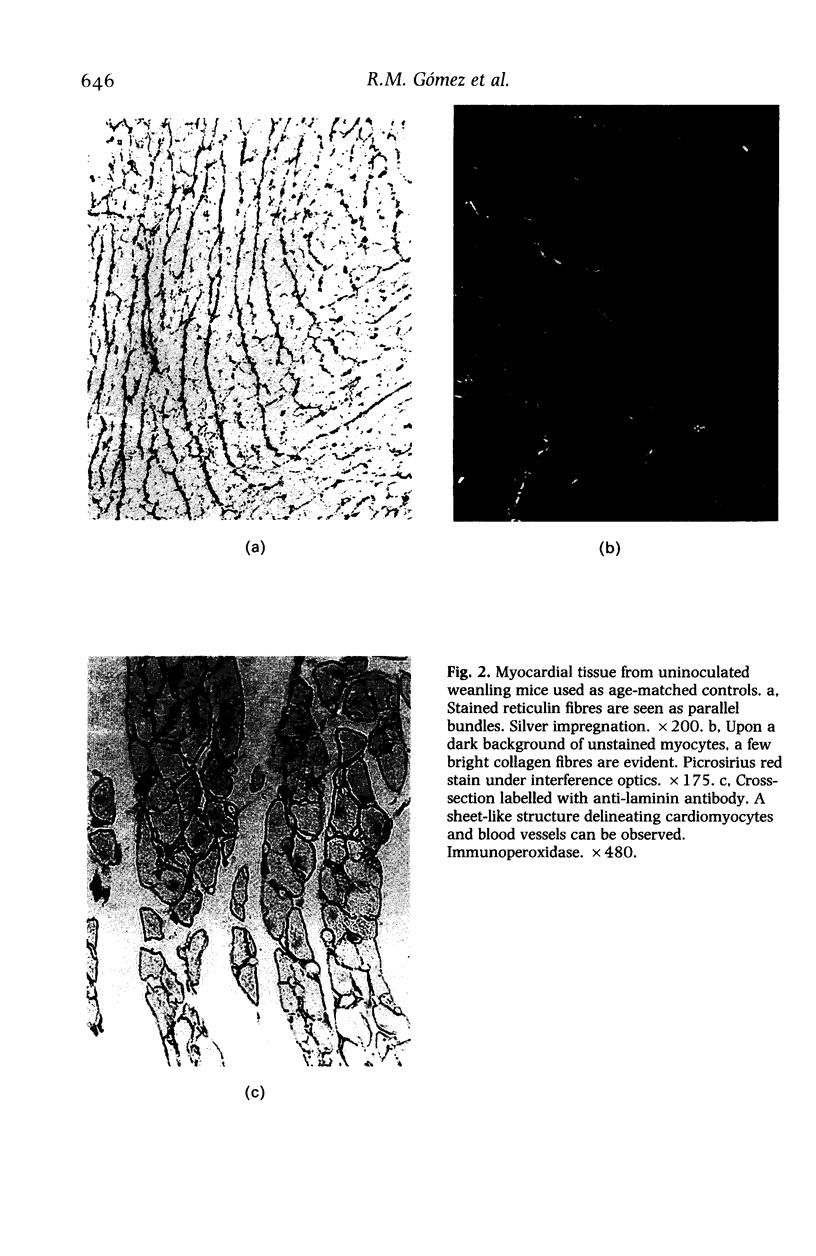
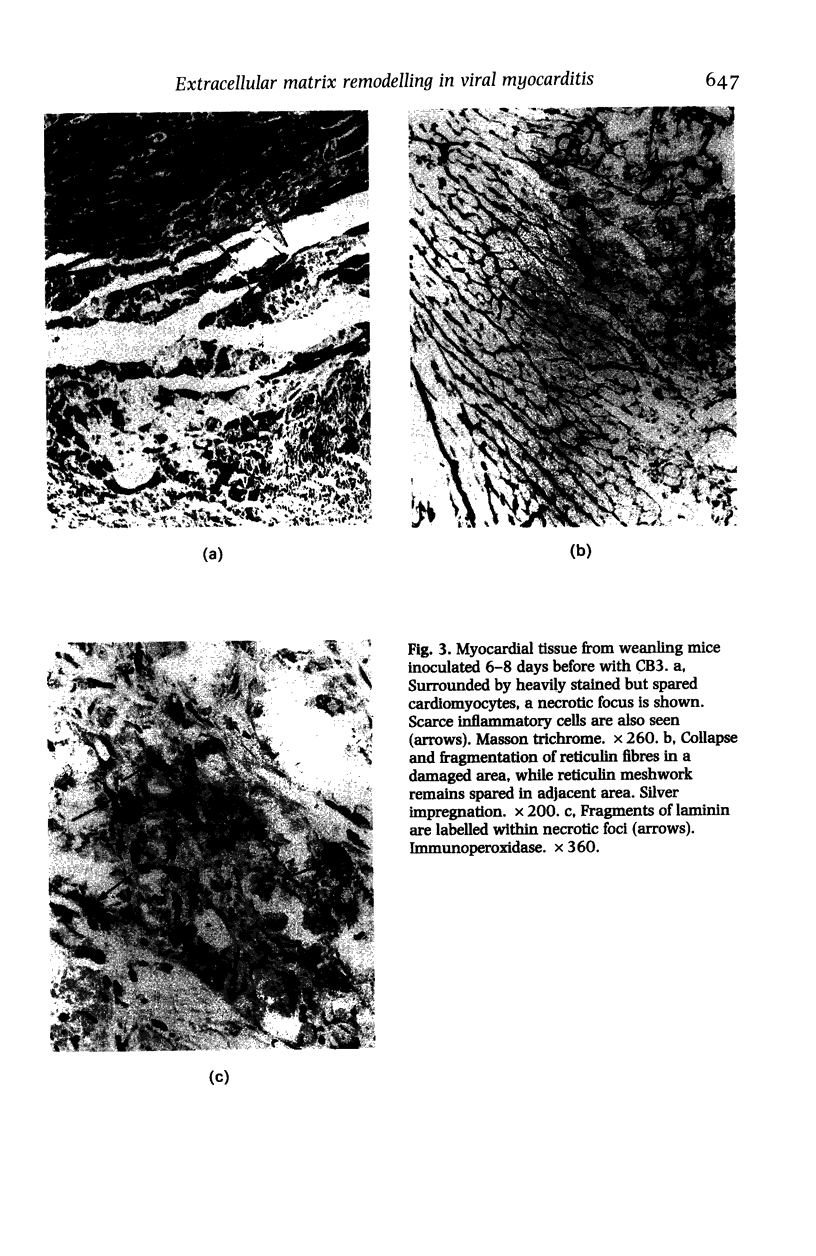
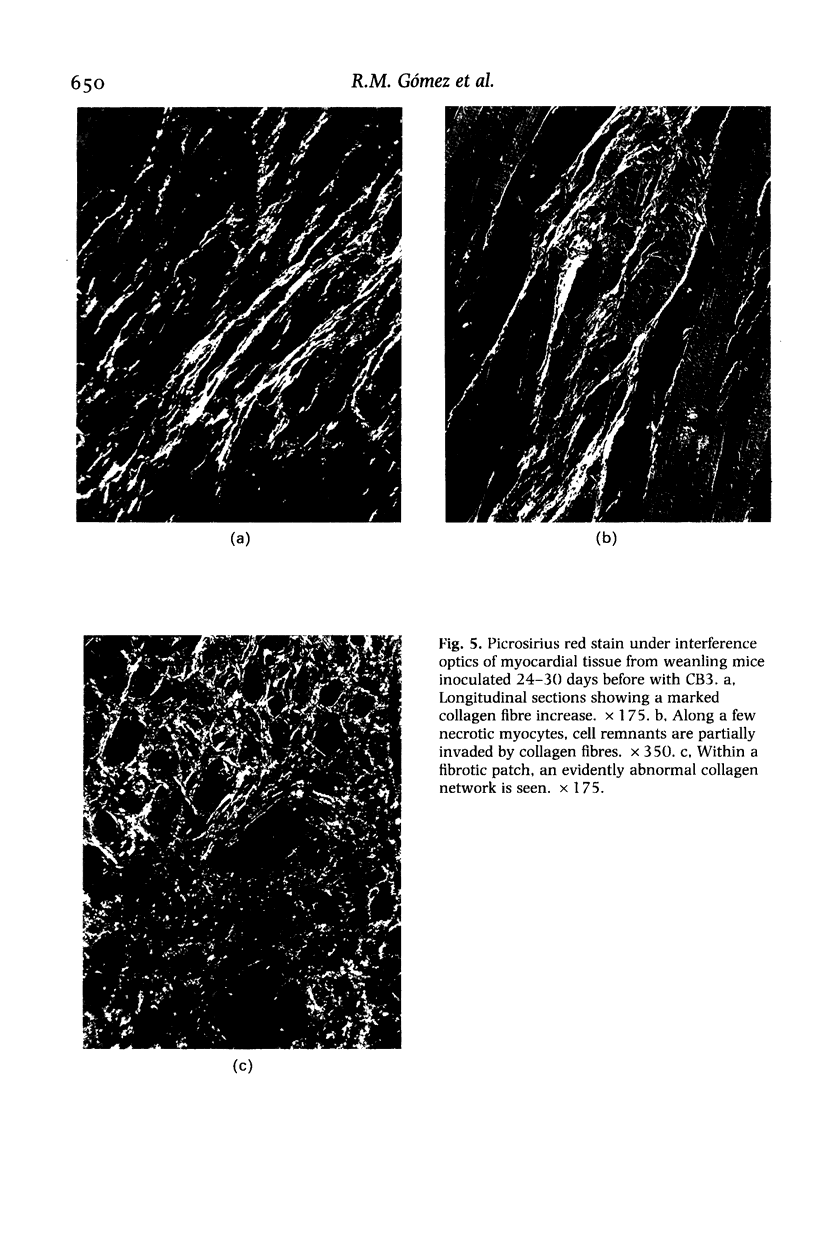
Abstract
Weanling inbred Balb/c mice were intraperitoneally inoculated with a myocarditic variant of coxsackievirus B3. At days 1, 2, 4, 6, 8, 10, 14, 24 and 30 post-infection (p.i.), myocardial tissue was harvested for viral infectivity titrations and histological studies, including routine techniques (haematoxylin-eosin, Masson trichrome and von Kossa) and specialized procedures (silver impregnation for reticulin, picrosirius red stain for collagen and immunoperoxidase labelling for laminin). Virus was isolated as from day 2, reached maximal infectivity at days 6-8 and decreased gradually to become undetectable by day 14. Early histological findings during the 1st week consisted mainly of scattered foci of necrotic myocytes showing calcium deposits; slight mononuclear cell infiltration and fragmentation of both reticulin fibres and pericellular laminin were also present. From the 2nd up to 4th week p.i., inflammatory reaction abated concomitantly with the gradual development of fibrosis, as evidenced by reticulin fibre thickening, irregular laminin distribution and collagen fibre increase. Our results suggest that viral-induced necrosis is able to trigger marked extracellular matrix remodelling even in the case of minimal inflammation.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. L., Carlquist J. F., Hammond E. H. Deficient natural killer cell activity in patients with idiopathic dilated cardiomyopathy. Lancet. 1982 Nov 20;2(8308):1124–1127. doi: 10.1016/s0140-6736(82)92786-6. [DOI] [PubMed] [Google Scholar]
- Anderson J. L., Carlquist J. F., Lutz J. R., DeWitt C. W., Hammond E. H. HLA A, B and DR typing in idiopathic dilated cardiomyopathy: a search for immune response factors. Am J Cardiol. 1984 May 1;53(9):1326–1330. doi: 10.1016/0002-9149(84)90088-2. [DOI] [PubMed] [Google Scholar]
- Bengtsson E., Lamberger B. Five-year follow-up study of cases suggestive of acute myocarditis. Am Heart J. 1966 Dec;72(6):751–763. doi: 10.1016/0002-8703(66)90158-x. [DOI] [PubMed] [Google Scholar]
- Bowles N. E., Richardson P. J., Olsen E. G., Archard L. C. Detection of Coxsackie-B-virus-specific RNA sequences in myocardial biopsy samples from patients with myocarditis and dilated cardiomyopathy. Lancet. 1986 May 17;1(8490):1120–1123. doi: 10.1016/s0140-6736(86)91837-4. [DOI] [PubMed] [Google Scholar]
- Contard F., Koteliansky V., Marotte F., Dubus I., Rappaport L., Samuel J. L. Specific alterations in the distribution of extracellular matrix components within rat myocardium during the development of pressure overload. Lab Invest. 1991 Jan;64(1):65–75. [PubMed] [Google Scholar]
- Eckstein R., Mempel W., Bolte H. D. Reduced suppressor cell activity in congestive cardiomyopathy and in myocarditis. Circulation. 1982 Jun;65(6):1224–1229. doi: 10.1161/01.cir.65.6.1224. [DOI] [PubMed] [Google Scholar]
- Edwards W. D. Cardiomyopathies. Hum Pathol. 1987 Jun;18(6):625–635. doi: 10.1016/s0046-8177(87)80364-7. [DOI] [PubMed] [Google Scholar]
- Eghbali M., Czaja M. J., Zeydel M., Weiner F. R., Zern M. A., Seifter S., Blumenfeld O. O. Collagen chain mRNAs in isolated heart cells from young and adult rats. J Mol Cell Cardiol. 1988 Mar;20(3):267–276. doi: 10.1016/s0022-2828(88)80059-2. [DOI] [PubMed] [Google Scholar]
- Gauntt C. J., Trousdale M. D., LaBadie D. R., Paque R. E., Nealon T. Properties of coxsackievirus B3 variants which are amyocarditic or myocarditic for mice. J Med Virol. 1979;3(3):207–220. doi: 10.1002/jmv.1890030307. [DOI] [PubMed] [Google Scholar]
- Goodwin J. F. Mechanisms in cardiomyopathies. J Mol Cell Cardiol. 1985 Jul;17 (Suppl 2):5–9. doi: 10.1016/0022-2828(85)90003-3. [DOI] [PubMed] [Google Scholar]
- Grun J. B., Schultz M., Finkelstein S. D., Crowell R. L., Landau B. J. Pathogenesis of acute myocardial necrosis in inbred mice infected with coxsackievirus B3. Microb Pathog. 1988 Jun;4(6):417–430. doi: 10.1016/0882-4010(88)90027-7. [DOI] [PubMed] [Google Scholar]
- Gómez R. M., Lascano E. F., Berría M. I. Murine acinar pancreatitis preceding necrotizing myocarditis after Coxsackievirus B3 inoculation. J Med Virol. 1991 Oct;35(2):71–75. doi: 10.1002/jmv.1890350202. [DOI] [PubMed] [Google Scholar]
- Jalil J. E., Doering C. W., Janicki J. S., Pick R., Shroff S. G., Weber K. T. Fibrillar collagen and myocardial stiffness in the intact hypertrophied rat left ventricle. Circ Res. 1989 Jun;64(6):1041–1050. doi: 10.1161/01.res.64.6.1041. [DOI] [PubMed] [Google Scholar]
- Jin O., Sole M. J., Butany J. W., Chia W. K., McLaughlin P. R., Liu P., Liew C. C. Detection of enterovirus RNA in myocardial biopsies from patients with myocarditis and cardiomyopathy using gene amplification by polymerase chain reaction. Circulation. 1990 Jul;82(1):8–16. doi: 10.1161/01.cir.82.1.8. [DOI] [PubMed] [Google Scholar]
- Junqueira L. C., Bignolas G., Brentani R. R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979 Jul;11(4):447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- Katz A. M., Freston J. W., Messineo F. C., Herbette L. G. Membrane damage and the pathogenesis of cardiomyopathies. J Mol Cell Cardiol. 1985 Jul;17 (Suppl 2):11–20. doi: 10.1016/0022-2828(85)90004-5. [DOI] [PubMed] [Google Scholar]
- Kawai C., Matsumori A., Fujiwara H. Myocarditis and dilated cardiomyopathy. Annu Rev Med. 1987;38:221–239. doi: 10.1146/annurev.me.38.020187.001253. [DOI] [PubMed] [Google Scholar]
- Kawai C., Matsumori A., Kumagai N., Tokuda M. Experimental Coxsackie virus B-3 and B-4 myocarditis in mice. Jpn Circ J. 1978 Jan;42(1):43–47. doi: 10.1253/jcj.42.43. [DOI] [PubMed] [Google Scholar]
- Kawai S., Shimizu M., Okada R., Ih S. A morphological analysis of chronic myocarditis. Jpn Circ J. 1987 Dec;51(12):1385–1392. doi: 10.1253/jcj.51.1385. [DOI] [PubMed] [Google Scholar]
- LASCANO E. F. Controlled differentiation of cells and connective tissue fibers in silver staining. Stain Technol. 1960 Jan;35:23–29. doi: 10.3109/10520296009114711. [DOI] [PubMed] [Google Scholar]
- Leslie K. O., Schwarz J., Simpson K., Huber S. A. Progressive interstitial collagen deposition in Coxsackievirus B3-induced murine myocarditis. Am J Pathol. 1990 Mar;136(3):683–693. [PMC free article] [PubMed] [Google Scholar]
- Leslie K., Blay R., Haisch C., Lodge A., Weller A., Huber S. Clinical and experimental aspects of viral myocarditis. Clin Microbiol Rev. 1989 Apr;2(2):191–203. doi: 10.1128/cmr.2.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren E., Gullberg D., Rubin K., Borg T. K., Terracio M. J., Terracio L. In vitro studies on adult cardiac myocytes: attachment and biosynthesis of collagen type IV and laminin. J Cell Physiol. 1988 Jul;136(1):43–53. doi: 10.1002/jcp.1041360106. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A., Delgado F. M., Amenta P. S. The extracellular matrix in hepatic regeneration. Localization of collagen types I, III, IV, laminin, and fibronectin. Lab Invest. 1991 Feb;64(2):157–166. [PubMed] [Google Scholar]
- Montague T. J., Lopaschuk G. D., Davies N. J. Viral heart disease. Chest. 1990 Jul;98(1):190–199. doi: 10.1378/chest.98.1.190. [DOI] [PubMed] [Google Scholar]
- Olsen E. G. The pathology of cardiomyopathies. A critical analysis. Am Heart J. 1979 Sep;98(3):385–392. doi: 10.1016/0002-8703(79)90052-8. [DOI] [PubMed] [Google Scholar]
- Rose N. R., Neumann D. A., Herskowitz A., Traystman M. D., Beisel K. W. Genetics of susceptibility to viral myocarditis in mice. Pathol Immunopathol Res. 1988;7(4):266–278. doi: 10.1159/000157122. [DOI] [PubMed] [Google Scholar]
- Sudhas-Na-Ayuthya P., Jayavasu J., Pongpanich B. Coxsackie group B virus and primary myocardial disease in infants and children. Am Heart J. 1974 Sep;88(3):311–314. doi: 10.1016/0002-8703(74)90464-5. [DOI] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Vracko R., Cunningham D., Frederickson R. G., Thorning D. Basal lamina of rat myocardium. Its fate after death of cardiac myocytes. Lab Invest. 1988 Jan;58(1):77–87. [PubMed] [Google Scholar]
- Wilson F. M., Miranda Q. R., Chason J. L., Lerner A. M. Residual pathologic changes following murine coxsackie A and B myocarditis. Am J Pathol. 1969 May;55(2):253–265. [PMC free article] [PubMed] [Google Scholar]
- Wolff P. G., Kühl U., Schultheiss H. P. Laminin distribution and autoantibodies to laminin in dilated cardiomyopathy and myocarditis. Am Heart J. 1989 Jun;117(6):1303–1309. doi: 10.1016/0002-8703(89)90410-9. [DOI] [PubMed] [Google Scholar]
- Woodruff J. F. Viral myocarditis. A review. Am J Pathol. 1980 Nov;101(2):425–484. [PMC free article] [PubMed] [Google Scholar]
- Zee-Cheng C. S., Tsai C. C., Palmer D. C., Codd J. E., Pennington D. G., Williams G. A. High incidence of myocarditis by endomyocardial biopsy in patients with idiopathic congestive cardiomyopathy. J Am Coll Cardiol. 1984 Jan;3(1):63–70. doi: 10.1016/s0735-1097(84)80431-3. [DOI] [PubMed] [Google Scholar]







