Abstract
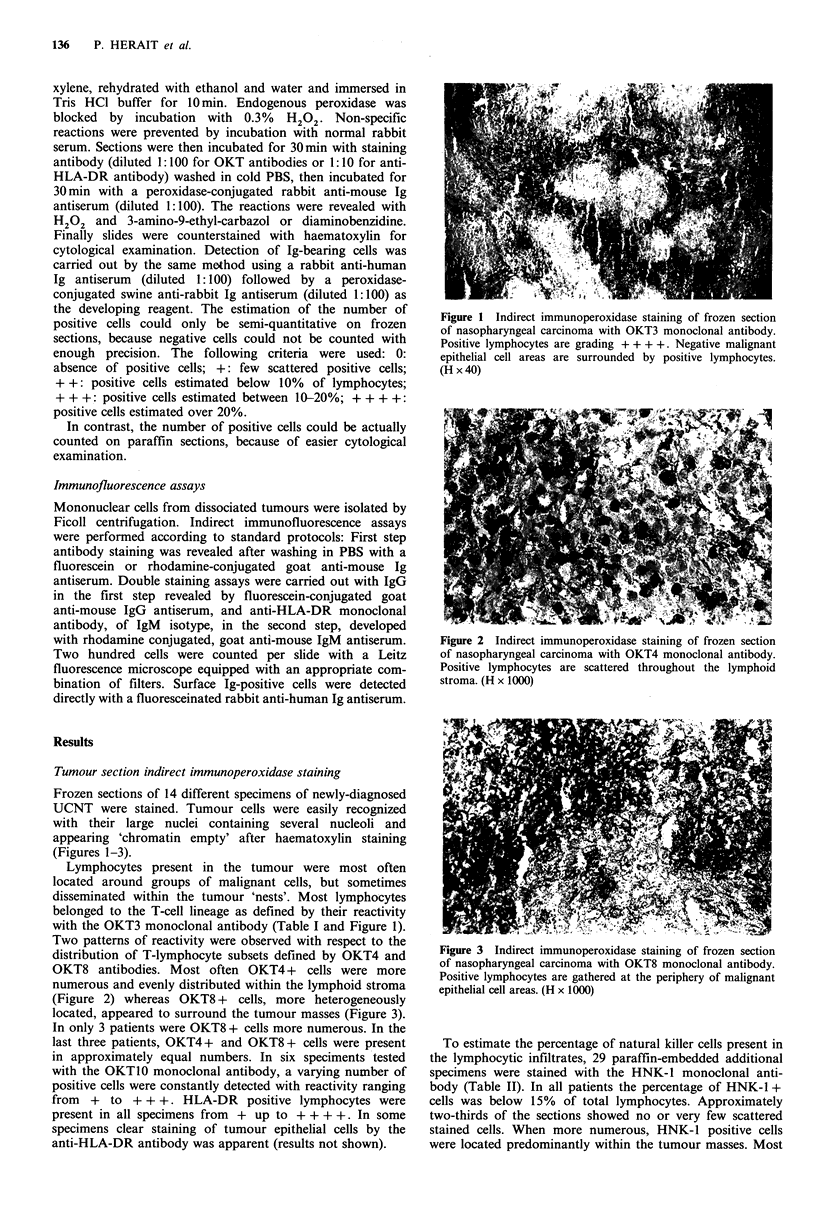
We have analyzed lymphocytes infiltrating nasopharyngeal carcinomas, using a combination of immunoperoxidase staining of frozen and paraffin-embedded sections, and immunofluorescence on lymphocyte suspensions recovered from teased tumours. A panel of monoclonal antibodies was used to define lymphocytic subsets on frozen sections of 14 different tumours. The vast majority of peri- and intra-tumoral lymphocytes were stained by OKT3 antibody. In 8 sections, T4 positive cells were largely predominant, while T8 positive cells were the majority in three sections. Twenty-nine paraffin-embedded sections from other NPC patients stained with HNK-1 antibody showed a variable percentage of positive cells reaching 6 to 15% in nine patients. Most HNK-1 positive cells had the morphology of large granular lymphocytes typical of natural killer cells. Double staining experiments on lymphocytes isolated from 7 tumours revealed a constant presence of T3 positive, HLA-DR positive lymphocytes (from 6 to 29% of mononuclear cells), and of lymphocytes coexpressing the T3 and the Tac (IL-2 receptor) antigens (from 5 to 12% of mononuclear cells). Lymphocytes with a phenotype of activated T-cells are thus constantly found in NPC tumours.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Balch C. M. A differentiation antigen of human NK and K cells identified by a monoclonal antibody (HNK-1). J Immunol. 1981 Sep;127(3):1024–1029. [PubMed] [Google Scholar]
- Caillaud J. M., Benjelloun S., Bosq J., Braham K., Lipinski M. HNK-1-defined antigen detected in paraffin-embedded neuroectoderm tumors and those derived from cells of the amine precursor uptake and decarboxylation system. Cancer Res. 1984 Oct;44(10):4432–4439. [PubMed] [Google Scholar]
- Galili U., Klein E., Klein G., Singh Bal I. Activated T lymphocytes in infiltrates and draining lymph nodes of nasopharyngeal carcinoma. Int J Cancer. 1980 Jan 15;25(1):85–89. doi: 10.1002/ijc.2910250111. [DOI] [PubMed] [Google Scholar]
- Henle G., Henle W. Epstein-Barr virus-specific IgA serum antibodies as an outstanding feature of nasopharyngeal carcinoma. Int J Cancer. 1976 Jan 15;17(1):1–7. doi: 10.1002/ijc.2910170102. [DOI] [PubMed] [Google Scholar]
- Henle W., Ho H. C., Henle G., Kwan H. C. Antibodies to Epstein-Barr virus-related antigens in nasopharyngeal carcinoma. Comparison of active cases with long-term survivors. J Natl Cancer Inst. 1973 Aug;51(2):361–369. [PubMed] [Google Scholar]
- Hennessy K., Fennewald S., Hummel M., Cole T., Kieff E. A membrane protein encoded by Epstein-Barr virus in latent growth-transforming infection. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7207–7211. doi: 10.1073/pnas.81.22.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herait P., Tursz T., Guillard M. Y., Hanna K., Lipinski M., Micheau C., Sancho-Garnier H., Schwaab G., Cachin Y., Degos L. HLA-A, -B, and -DR antigens in North African patients with nasopharyngeal carcinoma. Tissue Antigens. 1983 Nov;22(5):335–341. doi: 10.1111/j.1399-0039.1983.tb02262.x. [DOI] [PubMed] [Google Scholar]
- Hercend T., Ritz J., Schlossman S. F., Reinherz E. L. Comparative expression of T9, T10, and Ia antigens on activated human T cell subsets. Hum Immunol. 1981 Nov;3(3):247–259. doi: 10.1016/0198-8859(81)90021-5. [DOI] [PubMed] [Google Scholar]
- Lipinski M., Braham K., Caillaud J. M., Carlu C., Tursz T. HNK-1 antibody detects an antigen expressed on neuroectodermal cells. J Exp Med. 1983 Nov 1;158(5):1775–1780. doi: 10.1084/jem.158.5.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski M., Fridman W. H., Tursz T., Vincent C., Pious D., Fellous M. Absence of allogeneic restriction in human T-cell-mediated cytotoxicity to Epstein-Barr virus-infected target cells. Demonstration of an HLA-linked control at the effector level. J Exp Med. 1979 Dec 1;150(6):1310–1322. doi: 10.1084/jem.150.6.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss D. J., Chan S. H., Burrows S. R., Chew T. S., Kane R. G., Staples J. A., Kunaratnam N. Epstein-Barr virus specific T-cell response in nasopharyngeal carcinoma patients. Int J Cancer. 1983 Sep 15;32(3):301–305. doi: 10.1002/ijc.2910320307. [DOI] [PubMed] [Google Scholar]
- Pearson G. R., Johansson B., Klein G. Antibody-dependent cellular cytotoxicity against Epstein-Barr virus-associated antigens in African patients with nasopharyngeal carcinoma. Int J Cancer. 1978 Aug 15;22(2):120–125. doi: 10.1002/ijc.2910220203. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Pesando J. M., Ritz J., Goldstein G., Schlossman S. F. Ia determinants on human T-cell subsets defined by monoclonal antibody. Activation stimuli required for expression. J Exp Med. 1979 Dec 1;150(6):1472–1482. doi: 10.1084/jem.150.6.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickinson A. Epstein-Barr virus in epithelium. Nature. 1984 Jul 12;310(5973):99–100. doi: 10.1038/310099a0. [DOI] [PubMed] [Google Scholar]
- Saksela E., Timonen T., Ranki A., Häyry P. Morphological and functional characterization of isolated effector cells responsible for human natural killer activity to fetal fibroblasts and to cultured cell line targets. Immunol Rev. 1979;44:71–123. doi: 10.1111/j.1600-065x.1979.tb00268.x. [DOI] [PubMed] [Google Scholar]
- Shanmugaratnam K., Chan S. H., de-Thé G., Goh J. E., Khor T. H., Simons M. J., Tye C. Y. Histopathology of nasopharyngeal carcinoma: correlations with epidemiology, survival rates and other biological characteristics. Cancer. 1979 Sep;44(3):1029–1044. doi: 10.1002/1097-0142(197909)44:3<1029::aid-cncr2820440335>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Iliescu V., Crawford D. H., Ellouz R., Cammoun M., de-Thé G. Expression of HLA-DR antigens in nasopharyngeal carcinoma: an immunohistological analysis of the tumour cells and infiltrating lymphocytes. Int J Cancer. 1984 Jun 15;33(6):813–819. doi: 10.1002/ijc.2910330616. [DOI] [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]
- Wakasugi H., Bertoglio J., Tursz T., Fradelizi D. IL 2 receptor induction on human T lymphocytes: role for IL 2 and monocytes. J Immunol. 1985 Jul;135(1):321–327. [PubMed] [Google Scholar]
- Wolf H. Epstein-Barr virus and carcinoma. Nature. 1984 Dec 20;312(5996):705–705. doi: 10.1038/312705a0. [DOI] [PubMed] [Google Scholar]
- Wolf H., zur Hausen H., Becker V. EB viral genomes in epithelial nasopharyngeal carcinoma cells. Nat New Biol. 1973 Aug 22;244(138):245–247. doi: 10.1038/newbio244245a0. [DOI] [PubMed] [Google Scholar]
- de-Thé G., Ho J. H., Ablashi D. V., Day N. E., Macario A. J., Martin-Berthelon M. C., Pearson G., Sohier R. Nasopharyngeal carcinoma. IX. Antibodies to EBNA and correlation with response to other ebv antigens in chinese patients. Int J Cancer. 1975 Nov 15;16(5):713–721. doi: 10.1002/ijc.2910160503. [DOI] [PubMed] [Google Scholar]
- zur Hausen H., Schulte-Holthausen H., Klein G., Henle W., Henle G., Clifford P., Santesson L. EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature. 1970 Dec 12;228(5276):1056–1058. doi: 10.1038/2281056a0. [DOI] [PubMed] [Google Scholar]






