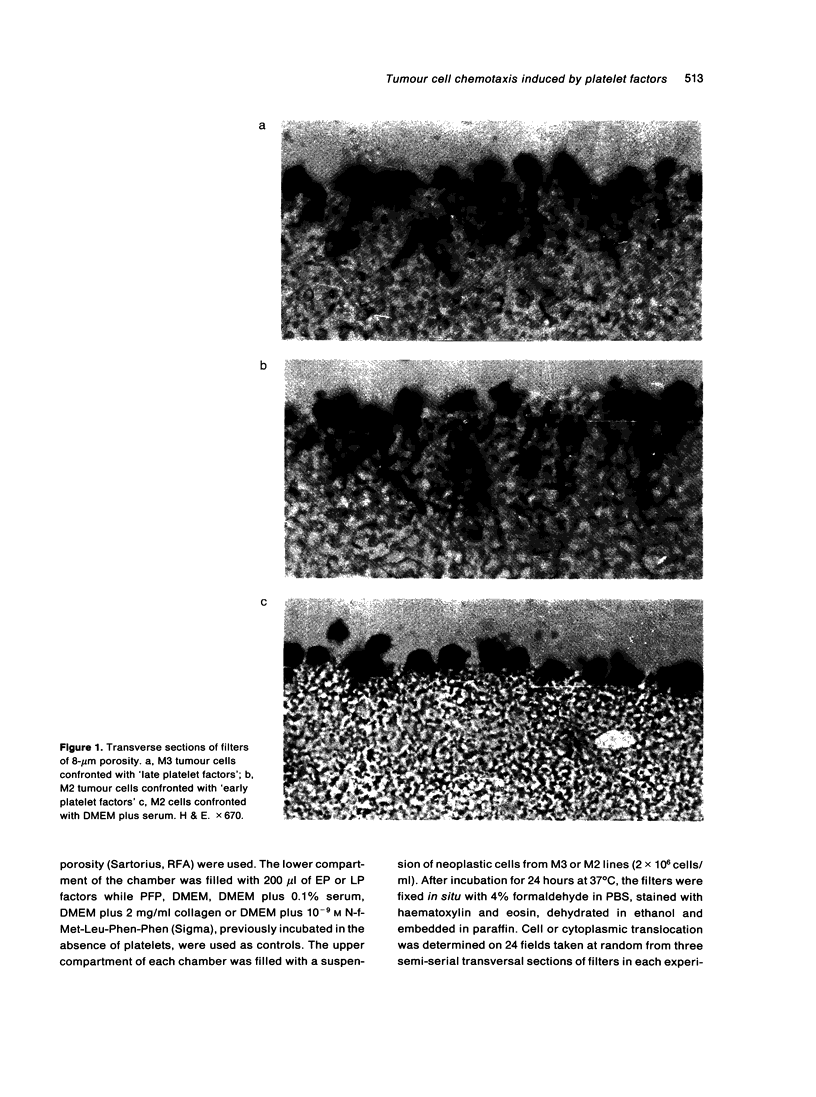
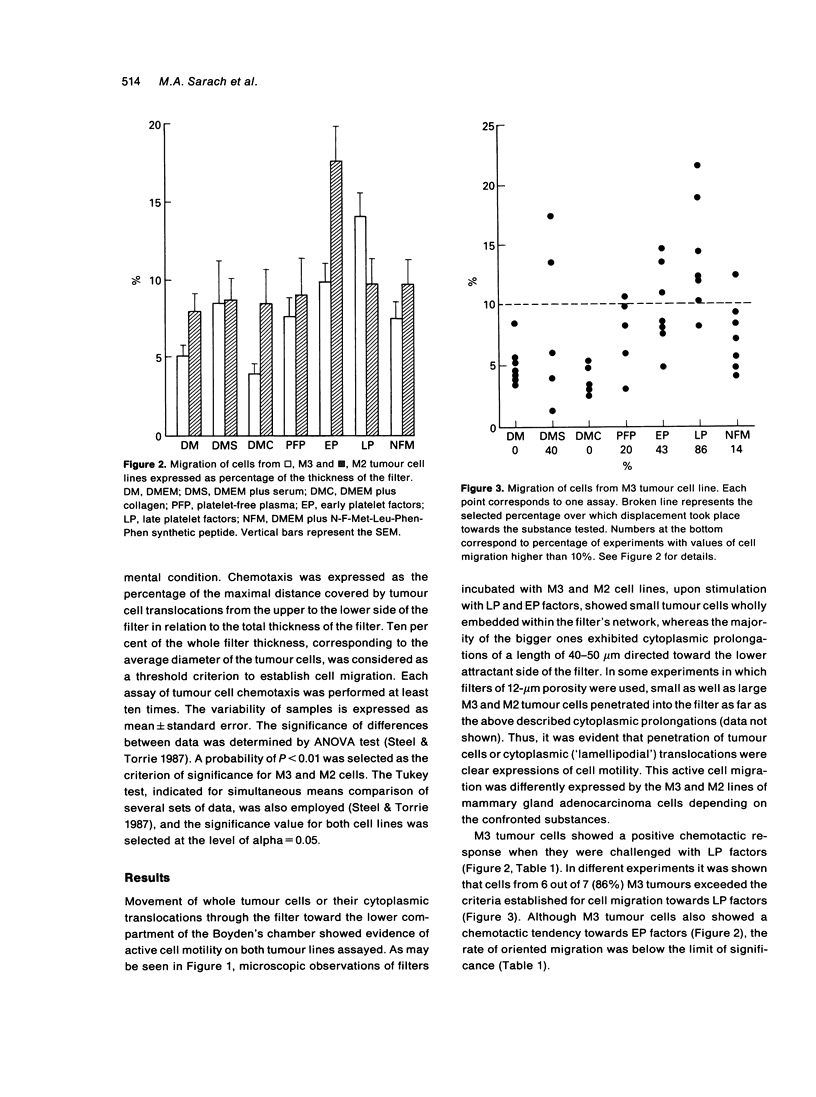
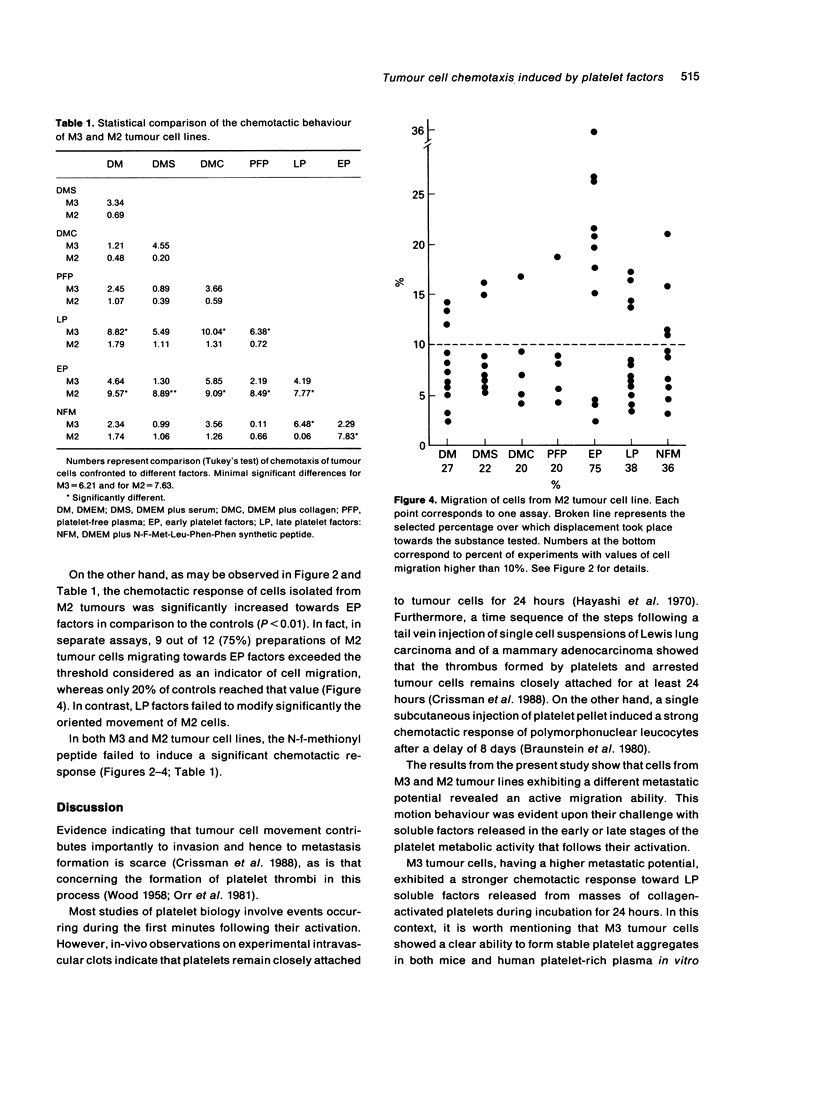
Abstract
The chemotactic response of neoplastic cells (NC) induced by soluble platelet factors was investigated. NC suspensions isolated from murine mammary gland adenocarcinomas having different metastatic capabilities were incubated in Boyden's chambers and challenged with (1) 'Early Platelet Factors' (EP), obtained from the soluble fraction of recently collagen-activated human platelets, and (2) 'Late Platelet Factors' (LP), isolated after 24 hours incubation of the platelet aggregates. Chemotaxis was expressed as the distance travelled by NC through nitrocellulose filters. NC isolated from M3, the tumour line having the stronger metastatic potential, showed a significant chemotactic response towards LP factors, whereas NC from the M2 line exhibiting the lower metastatic behaviour, showed a chemotactic response towards EP factors. Both tumour cell lines lacked motion capability towards the well known chemoattractant peptide N-f-Met-Leu-Phe-Phe as well as to serum, plasma, collagen type I or culture medium. The different chemotactic response of both tumour lines when they were challenged by concentration gradients of factors released by early or late collagen-activated human platelets, confirm a relationship between platelet activity and metastatic capabilities and suggests that platelet chemoattractants might play a role in the metastatic dissemination of these mammary gland adenocarcinomas.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allam M., Martinet N., Martinet Y. Differential migratory response of U-2 OS osteosarcoma cell to the various forms of platelet-derived growth factor. Biochimie. 1992 Feb;74(2):183–186. doi: 10.1016/0300-9084(92)90044-f. [DOI] [PubMed] [Google Scholar]
- Braunstein P. W., Cuénoud H. F., Joris I., Majno G. Platelets, fibroblasts, and inflammation: tissue reactions to platelets injected subcutaneously. Am J Pathol. 1980 Apr;99(1):53–66. [PMC free article] [PubMed] [Google Scholar]
- Crissman J. D., Hatfield J. S., Menter D. G., Sloane B., Honn K. V. Morphological study of the interaction of intravascular tumor cells with endothelial cells and subendothelial matrix. Cancer Res. 1988 Jul 15;48(14):4065–4072. [PubMed] [Google Scholar]
- Eynard A. R., Falo C., Quiroga P., Pasqualini M. E. In vitro interaction of heterologous platelets with isolated neoplastic cells from a murine mammary gland tumor. Exp Cell Biol. 1989;57(4):213–218. doi: 10.1159/000163528. [DOI] [PubMed] [Google Scholar]
- Eynard A. R., Pasqualini M. E., Rovasio R. A. Exogenous fibronectin modifies the aggregation of collagen-stimulated human platelets. Experientia. 1990 Jul 15;46(7):680–682. doi: 10.1007/BF01939932. [DOI] [PubMed] [Google Scholar]
- Goetzl E. J., Pickett W. C. The human PMN leukocyte chemotactic activity of complex hydroxy-eicosatetraenoic acids (HETEs). J Immunol. 1980 Oct;125(4):1789–1791. [PubMed] [Google Scholar]
- Grimstad I. A. Direct evidence that cancer cell locomotion contributes importantly to invasion. Exp Cell Res. 1987 Dec;173(2):515–523. doi: 10.1016/0014-4827(87)90291-6. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Yoshida K., Ozaki T., Ushijima K. Chemotactic factor associated with invasion of cancer cells. Nature. 1970 Apr 11;226(5241):174–175. doi: 10.1038/226174a0. [DOI] [PubMed] [Google Scholar]
- König W., Bremm K. D., Müller P., Kunau W. H., Borgeat P., Spur B., Crea A. E., Falsone G. On the biological role of lipid chemotactic factors. Agents Actions Suppl. 1983;12:167–185. doi: 10.1007/978-3-0348-9352-7_10. [DOI] [PubMed] [Google Scholar]
- Lagarde M. Metabolism of fatty acids by platelets and the functions of various metabolites in mediating platelet function. Prog Lipid Res. 1988;27(2):135–152. doi: 10.1016/0163-7827(88)90008-2. [DOI] [PubMed] [Google Scholar]
- Liapi C., Raynaud F., Anderson W. B., Evain-Brion D. High chemotactic response to platelet-derived growth factor of a teratocarcinoma differentiated mesodermal cell line. In Vitro Cell Dev Biol. 1990 Apr;26(4):388–392. doi: 10.1007/BF02623830. [DOI] [PubMed] [Google Scholar]
- Mezzano S., Burgos M. E., Ardiles L., Olavarria F., Concha M., Caorsi I., Aranda E., Mezzano D. Glomerular localization of platelet factor 4 in streptococcal nephritis. Nephron. 1992;61(1):58–63. doi: 10.1159/000186835. [DOI] [PubMed] [Google Scholar]
- Mooradian D. L., McCarthy J. B., Komanduri K. V., Furcht L. T. Effects of transforming growth factor-beta 1 on human pulmonary adenocarcinoma cell adhesion, motility, and invasion in vitro. J Natl Cancer Inst. 1992 Apr 1;84(7):523–527. doi: 10.1093/jnci/84.7.523. [DOI] [PubMed] [Google Scholar]
- Newgreen D. F. Physical influences on neural crest cell migration in avian embryos: contact guidance and spatial restriction. Dev Biol. 1989 Jan;131(1):136–148. doi: 10.1016/s0012-1606(89)80045-4. [DOI] [PubMed] [Google Scholar]
- Orr F. W., Varani J., Delikatny J., Jain N., Ward P. A. Comparison of the chemotactic responsiveness of two fibrosarcoma subpopulations of differing malignancy. Am J Pathol. 1981 Feb;102(2):160–167. [PMC free article] [PubMed] [Google Scholar]
- Orr F. W., Varani J., Gondek M. D., Ward P. A., Mundy G. R. Partial characterization of a bone-derived chemotactic factor for tumor cells. Am J Pathol. 1980 Apr;99(1):43–52. [PMC free article] [PubMed] [Google Scholar]
- Orr W., Varani J., Ward P. A. Characteristics of the chemotactic response of neoplastic cells to a factor derived from the fifth component of complement. Am J Pathol. 1978 Nov;93(2):405–422. [PMC free article] [PubMed] [Google Scholar]
- Romualdez A. G., Jr, Ward P. A. A unique complement derived chemotactic factor for tumor cells. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4128–4132. doi: 10.1073/pnas.72.10.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Harker L. Hyperlipidemia and atherosclerosis. Science. 1976 Sep 17;193(4258):1094–1100. doi: 10.1126/science.822515. [DOI] [PubMed] [Google Scholar]
- Showell H. J., Freer R. J., Zigmond S. H., Schiffmann E., Aswanikumar S., Corcoran B., Becker E. L. The structure-activity relations of synthetic peptides as chemotactic factors and inducers of lysosomal secretion for neutrophils. J Exp Med. 1976 May 1;143(5):1154–1169. doi: 10.1084/jem.143.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spearman M. A., Ballon B. C., Gerrard J. M., Greenberg A. H., Wright J. A. The inhibition of platelet aggregation of metastatic H-ras-transformed 10T1/2 fibroblasts with castanospermine, an N-linked glycoprotein processing inhibitor. Cancer Lett. 1991 Dec 1;60(3):185–191. doi: 10.1016/0304-3835(91)90112-u. [DOI] [PubMed] [Google Scholar]
- Taraboletti G., Roberts D. D., Liotta L. A. Thrombospondin-induced tumor cell migration: haptotaxis and chemotaxis are mediated by different molecular domains. J Cell Biol. 1987 Nov;105(5):2409–2415. doi: 10.1083/jcb.105.5.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner S. R., Tainer J. A., Lynn W. S. Biogenesis of chemotactic molecules by the arachidonate lipoxygenase system of platelets. Nature. 1975 Oct 23;257(5528):680–681. doi: 10.1038/257680a0. [DOI] [PubMed] [Google Scholar]
- WOOD S., Jr Pathogenesis of metastasis formation observed in vivo in the rabbit ear chamber. AMA Arch Pathol. 1958 Oct;66(4):550–568. [PubMed] [Google Scholar]
- Wass J. A., Varani J., Piontek G. E., Goff D., Ward P. A. Characteristics of the chemotactic factor-mediated cell swelling response of tumor cells. J Natl Cancer Inst. 1981 May;66(5):927–933. [PubMed] [Google Scholar]
- Weiss L. A pathobiologic overview of metastasis. Semin Oncol. 1977 Mar;4(1):5–17. [PubMed] [Google Scholar]
- Weksler B. B., Coupal C. E. Platelet-dependent generation of chemotactic activity in serum. J Exp Med. 1973 Jun 1;137(6):1419–1430. doi: 10.1084/jem.137.6.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. Y., Purdie A. T., Han P. Thrombospondin and other possible related matrix proteins in malignant and benign breast disease. An immunohistochemical study. Am J Pathol. 1992 Jun;140(6):1473–1482. [PMC free article] [PubMed] [Google Scholar]
- Yabkowitz R., Dixit V. M. Human carcinoma cells express receptors for distinct domains of thrombospondin. Cancer Res. 1991 Mar 15;51(6):1645–1650. [PubMed] [Google Scholar]