Abstract
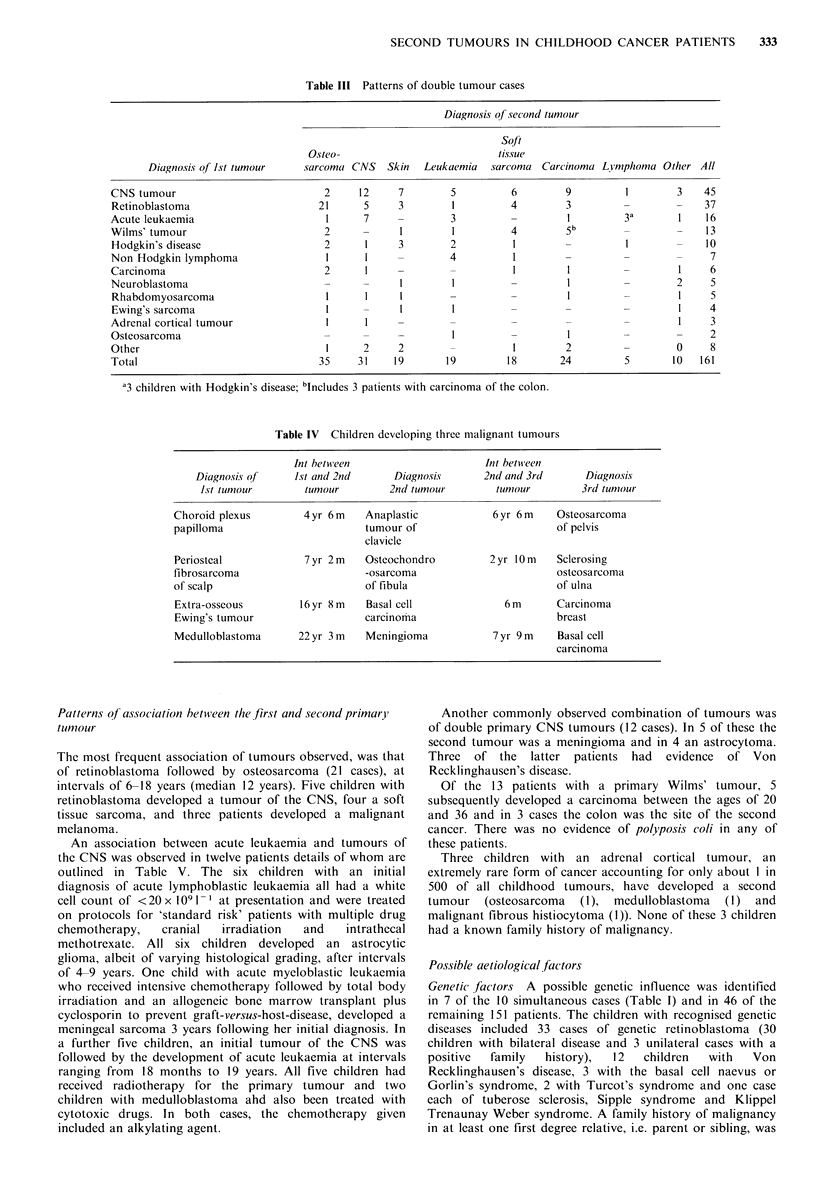
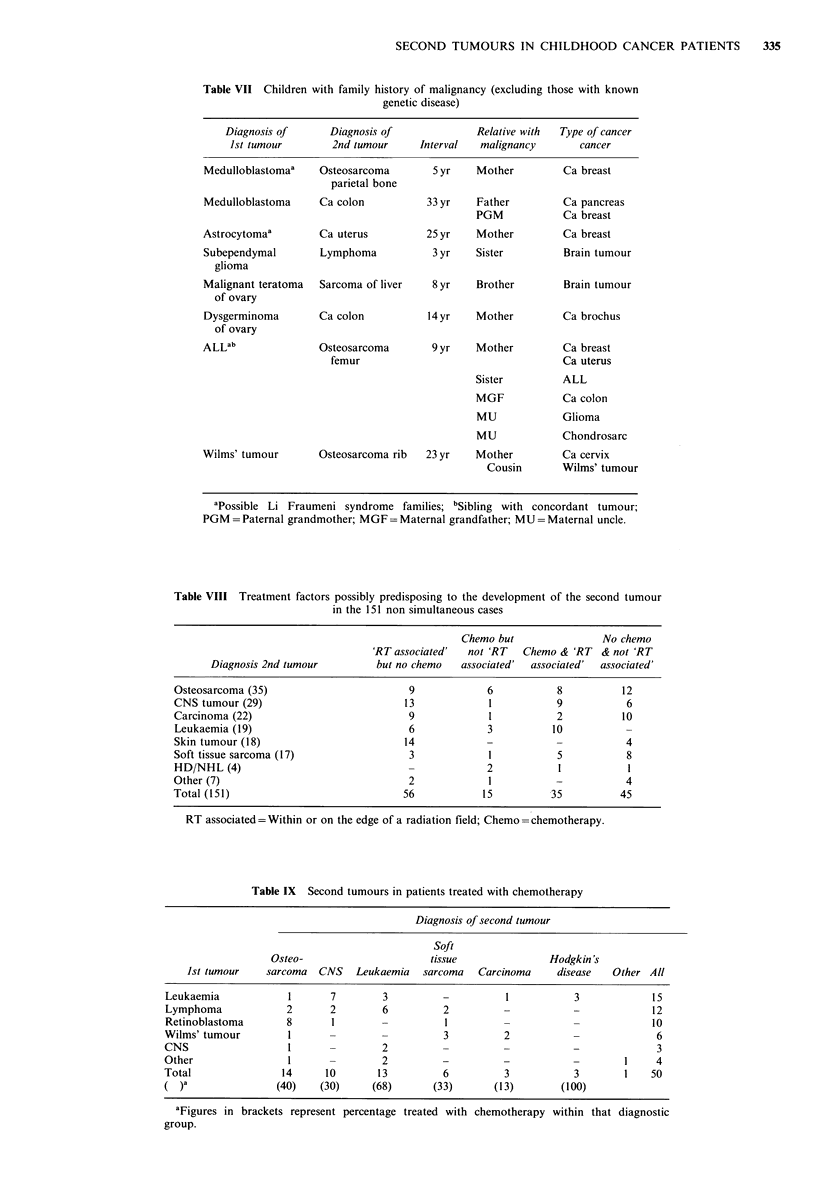
One hundred and sixty one children who have developed more than one primary neoplasm have been identified. Children with tumours of the central nervous system, retinoblastoma and leukaemia were those most frequently observed to develop a second malignancy whilst osteosarcoma was the most common second tumour. The patterns of second neoplasms appear to be changing and a recent increase in the number of children with leukaemia and lymphoma who develop second primary tumours has been observed. In this series, the two most frequent associations of tumours were retinoblastoma followed by osteosarcoma and the combination of acute leukaemia with a tumour of the central nervous system. Genetic factors which may have contributed to the development of the second primary tumour were identified in 53 patients (33%), 33 of whom had the genetic form of retinoblastoma. In an analysis of the treatment of 151 patients, for whom the interval between the two neoplasms was greater than 12 months, the second malignancy was considered to be 'radiation associated' in 93 (61%). Fifty children (33%) had been treated with either single or multiple agent chemotherapy which included an alkylating agent in 38. Forty five children had received a combination of chemotherapy and radiotherapy and of these, 10 developed leukaemia as their second tumour. Of the 19 secondary leukaemias, 16 have occurred in patients treated since 1970.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson D. H., Ellsworth R. M., Kitchin F. D., Tung G. Second nonocular tumors in retinoblastoma survivors. Are they radiation-induced? Ophthalmology. 1984 Nov;91(11):1351–1355. doi: 10.1016/s0161-6420(84)34127-6. [DOI] [PubMed] [Google Scholar]
- Abramson D. H., Ellsworth R. M., Zimmerman L. E. Nonocular cancer in retinoblastoma survivors. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1976 May-Jun;81(3 Pt 1):454–457. [PubMed] [Google Scholar]
- Anderson J. R., Treip C. S. Radiation-induced intracranial neoplasms. A report of three possible cases. Cancer. 1984 Feb 1;53(3):426–429. doi: 10.1002/1097-0142(19840201)53:3<426::aid-cncr2820530310>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Arseneau J. C., Canellos G. P., Johnson R., DeVita V. T., Jr Risk of new cancers in patients with Hodgkin's disease. Cancer. 1977 Oct;40(4 Suppl):1912–1916. doi: 10.1002/1097-0142(197710)40:4+<1912::aid-cncr2820400823>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Bonnin J. M., Rubinstein L. J., Palmer N. F., Beckwith J. B. The association of embryonal tumors originating in the kidney and in the brain. A report of seven cases. Cancer. 1984 Nov 15;54(10):2137–2146. doi: 10.1002/1097-0142(19841115)54:10<2137::aid-cncr2820541014>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Cadman E. C., Capizzi R. L., Bertino J. R. Acute nonlymphocytic leukemia: a delayed complication of Hodgkin's disease therapy: analysis of 109 cases. Cancer. 1977 Sep;40(3):1280–1296. doi: 10.1002/1097-0142(197709)40:3<1280::aid-cncr2820400343>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Curtin C. T., McHeffy B., Kolarsick A. J. Thyroid and breast cancer following childhood radiation. Cancer. 1977 Dec;40(6):2911–2913. doi: 10.1002/1097-0142(197712)40:6<2911::aid-cncr2820400622>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- DerKinderen D. J., Koten J. W., Den Otter W., Tan K. E., Beemer F. A. Retinoblastoma, melanoma, and pancreatic cancer. Lancet. 1986 Dec 6;2(8519):1335–1336. doi: 10.1016/s0140-6736(86)91463-7. [DOI] [PubMed] [Google Scholar]
- Draper G. J., Sanders B. M., Kingston J. E. Second primary neoplasms in patients with retinoblastoma. Br J Cancer. 1986 May;53(5):661–671. doi: 10.1038/bjc.1986.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farwell J., Flannery J. T. Cancer in relatives of children with central-nervous-system neoplasms. N Engl J Med. 1984 Sep 20;311(12):749–753. doi: 10.1056/NEJM198409203111201. [DOI] [PubMed] [Google Scholar]
- Farwell J., Flannery J. T. Second primaries in children with central nervous system tumors. J Neurooncol. 1984;2(4):371–375. doi: 10.1007/BF00178120. [DOI] [PubMed] [Google Scholar]
- Hawkins M. M., Draper G. J., Kingston J. E. Incidence of second primary tumours among childhood cancer survivors. Br J Cancer. 1987 Sep;56(3):339–347. doi: 10.1038/bjc.1987.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L., Mott M. G., Mann J. R., Raafat F., Darbyshire P. J., Morris Jones P. H. Second malignancies in children treated for non-Hodgkin's lymphoma and T-cell leukaemia with the UKCCSG regimens. Br J Cancer. 1987 Apr;55(4):463–466. doi: 10.1038/bjc.1987.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jick H., Walker A. M., Rothman K. J. The epidemic of endometrial cancer: a commentary. Am J Public Health. 1980 Mar;70(3):264–267. doi: 10.2105/ajph.70.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge M. R., Eden O. B., O'Neill P. Cerebral glioma after cranial prophylaxis for acute lymphoblastic leukaemia. Br Med J (Clin Res Ed) 1984 Oct 20;289(6451):1038–1039. doi: 10.1136/bmj.289.6451.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston J. E., Plowman P. N., Hungerford J. L. Ectopic intracranial retinoblastoma in childhood. Br J Ophthalmol. 1985 Oct;69(10):742–748. doi: 10.1136/bjo.69.10.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koriech O. M., McNaught G. H. Second primary neoplasm in a dysgerminoma patient. Br J Radiol. 1981 Nov;54(647):1005–1005. doi: 10.1259/0007-1285-54-647-1005. [DOI] [PubMed] [Google Scholar]
- Lee W. R., Laurie J., Townsend A. L. Fine structure of a radiation-induced osteogenic sarcoma. Cancer. 1975 Oct;36(4):1414–1425. doi: 10.1002/1097-0142(197510)36:4<1414::aid-cncr2820360433>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Li F. P., Fraumeni J. F., Jr Soft-tissue sarcomas, breast cancer, and other neoplasms. A familial syndrome? Ann Intern Med. 1969 Oct;71(4):747–752. doi: 10.7326/0003-4819-71-4-747. [DOI] [PubMed] [Google Scholar]
- Li F. P. Second malignant tumors after cancer in childhood. Cancer. 1977 Oct;40(4 Suppl):1899–1902. doi: 10.1002/1097-0142(197710)40:4+<1899::aid-cncr2820400821>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Meadows A. T., Baum E., Fossati-Bellani F., Green D., Jenkin R. D., Marsden B., Nesbit M., Newton W., Oberlin O., Sallan S. G. Second malignant neoplasms in children: an update from the Late Effects Study Group. J Clin Oncol. 1985 Apr;3(4):532–538. doi: 10.1200/JCO.1985.3.4.532. [DOI] [PubMed] [Google Scholar]
- Meadows A. T., D'Angio G. J., Miké V., Banfi A., Harris C., Jenkin R. D., Schwartz A. Patterns of second malignant neoplasms in children. Cancer. 1977 Oct;40(4 Suppl):1903–1911. doi: 10.1002/1097-0142(197710)40:4+<1903::aid-cncr2820400822>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Meadows A. T., Strong L. C., Li F. P., D'Angio G. J., Schweisguth O., Freeman A. I., Jenkin R. D., Morris-Jones P., Nesbit M. E. Bone sarcoma as a second malignant neoplasm in children: influence of radiation and genetic predisposition for the Late Effects Study Group. Cancer. 1980 Dec 15;46(12):2603–2606. doi: 10.1002/1097-0142(19801215)46:12<2603::aid-cncr2820461212>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Miké V., Meadows A. T., D'Angio G. J. Incidence of second malignant neoplasms in children: results of an international study. Lancet. 1982 Dec 11;2(8311):1326–1331. doi: 10.1016/s0140-6736(82)91524-0. [DOI] [PubMed] [Google Scholar]
- Modan B., Baidatz D., Mart H., Steinitz R., Levin S. G. Radiation-induced head and neck tumours. Lancet. 1974 Feb 23;1(7852):277–279. doi: 10.1016/s0140-6736(74)92592-6. [DOI] [PubMed] [Google Scholar]
- Mosijczuk A. D., Ruymann F. B. Second malignancy in acute lymphocytic leukemia. Review of 33 cases. Am J Dis Child. 1981 Apr;135(4):313–316. doi: 10.1001/archpedi.1981.02130280003002. [DOI] [PubMed] [Google Scholar]
- Mulvihill J. J., McKeen E. A. Discussion: genetics of multiple primary tumors: a clinical etiologic approach illustrated by three patients. Cancer. 1977 Oct;40(4 Suppl):1867–1871. doi: 10.1002/1097-0142(197710)40:4+<1867::aid-cncr2820400816>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Pearson A. D., Craft A. W., Perry R. H., Kalbag R. M., Evans R. G. Four primary tumors in one child. Cancer. 1983 Dec 15;52(12):2363–2368. doi: 10.1002/1097-0142(19831215)52:12<2363::aid-cncr2820521234>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Prentice A. G., Smith A. G., Bradstock K. F. Mixed lymphoblastic-myelomonoblastic leukemia in treated Hodgkin's disease. Blood. 1980 Jul;56(1):129–133. [PubMed] [Google Scholar]
- Risdall R. J., McKenna R. W., Nesbit M. E., Krivit W., Balfour H. H., Jr, Simmons R. L., Brunning R. D. Virus-associated hemophagocytic syndrome: a benign histiocytic proliferation distinct from malignant histiocytosis. Cancer. 1979 Sep;44(3):993–1002. doi: 10.1002/1097-0142(197909)44:3<993::aid-cncr2820440329>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Schmähl D., Habs M., Lorenz M., Wagner I. Occurrence of second tumors in man after anticancer drug treatment. Cancer Treat Rev. 1982 Sep;9(3):167–194. doi: 10.1016/s0305-7372(82)80006-6. [DOI] [PubMed] [Google Scholar]
- Secker-Walker L. M., Stewart E. L., Todd A. Acute lymphoblastic leukaemia with t(4;11) follows neuroblastoma: a late effect of treatment? Med Pediatr Oncol. 1985;13(1):48–50. doi: 10.1002/mpo.2950130112. [DOI] [PubMed] [Google Scholar]
- Stevenson J. C., Hillyard C. J., Spanos E., MacIntyre I., Ackroyd N., Lynn J., Brown M. J., Stevenson B. M. Sipple syndrome: marked variability of the disease within a family and implications for management. Postgrad Med J. 1981 Feb;57(664):104–108. doi: 10.1136/pgmj.57.664.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong L. C., Herson J., Osborne B. M., Sutow W. W. Risk of radiation-related subsequent malignant tumors in survivors of Ewing's sarcoma. J Natl Cancer Inst. 1979 Jun;62(6):1401–1406. [PubMed] [Google Scholar]


