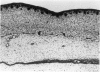
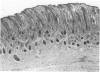
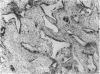
Abstract
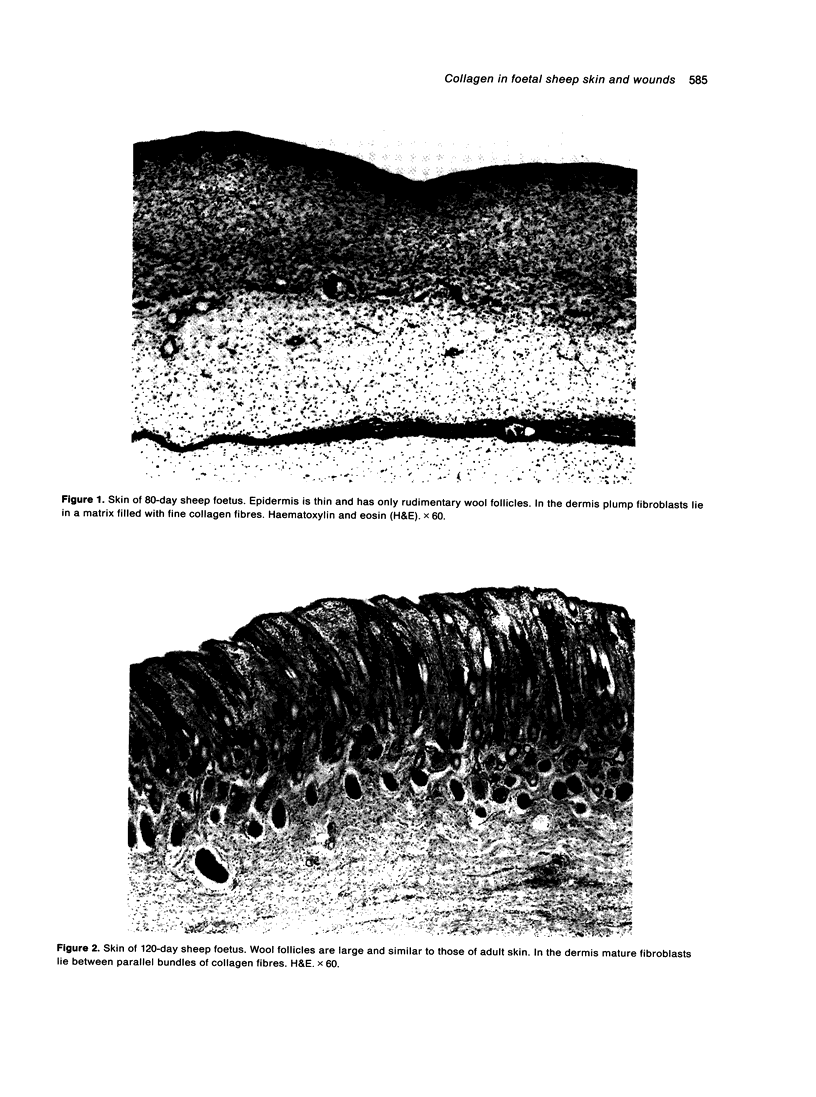
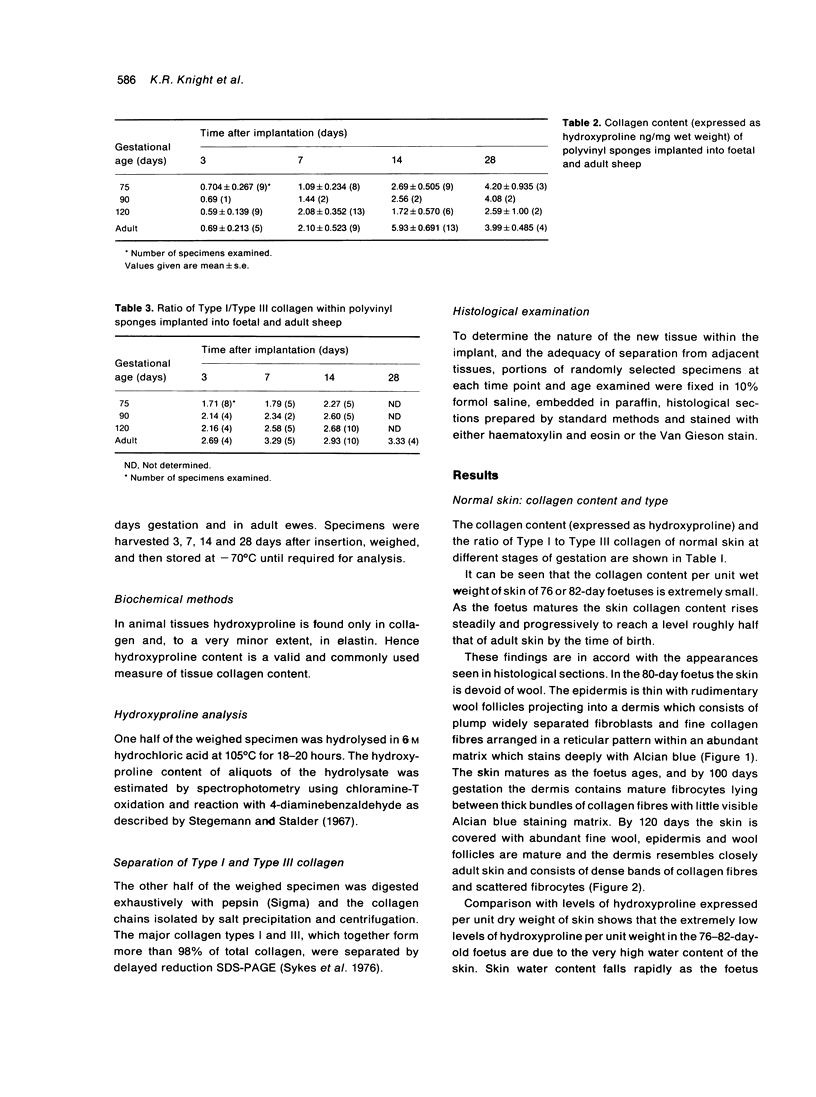
Total collagen content (measured as hydroxyproline) and Type I/Type III ratio (measured by SDS-PAGE) of normal skin and of scar tissue developing within a subcutaneously implanted polyvinyl sponge have been determined in 75, 90 and 120-day foetal lambs and adult sheep and correlated with histological appearances of the same tissues. Collagen content of normal skin is low at 75 days and rises progressively until birth when it is about half the adult level. The proportion of Type III in normal skin is highest at 75 days and falls progressively as the foetus develops. With implanted sponges the time course of changes in collagen content and I/III ratio are similar in all foetal groups and in adult sheep. Collagen content is low 3 days after implantation and rises progressively to reach a similar level in all groups by 28 days. The levels correlate closely with the amount of collagen visible in histological sections. The proportion of Type III is highest at 3 days in all groups and falls progressively as the newly formed tissue matures. The findings confirm our previous study of the healing of skin wounds that form as early as 75 days gestation foetal lambs can form scar tissue in a similar way to adult sheep.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adzick N. S., Harrison M. R., Glick P. L., Beckstead J. H., Villa R. L., Scheuenstuhl H., Goodson W. H., 3rd Comparison of fetal, newborn, and adult wound healing by histologic, enzyme-histochemical, and hydroxyproline determinations. J Pediatr Surg. 1985 Aug;20(4):315–319. doi: 10.1016/s0022-3468(85)80210-4. [DOI] [PubMed] [Google Scholar]
- Burd D. A., Longaker M. T., Adzick N. S., Harrison M. R., Ehrlich H. P. Foetal wound healing in a large animal model: the deposition of collagen is confirmed. Br J Plast Surg. 1990 Sep;43(5):571–577. doi: 10.1016/0007-1226(90)90122-g. [DOI] [PubMed] [Google Scholar]
- Burrington J. D. Wound healing in the fetal lamb. J Pediatr Surg. 1971 Oct;6(5):523–528. doi: 10.1016/0022-3468(71)90373-3. [DOI] [PubMed] [Google Scholar]
- Epstein E. H., Jr (Alpha1(3))3 human skin collagen. Release by pepsin digestion and preponderance in fetal life. J Biol Chem. 1974 May 25;249(10):3225–3231. [PubMed] [Google Scholar]
- HESS A. Reactions of mammalian fetal tissues to injury. II. Skin. Anat Rec. 1954 Aug;119(4):435–447. doi: 10.1002/ar.1091190404. [DOI] [PubMed] [Google Scholar]
- Hallock G. G. In utero cleft lip repair in A/J mice. Plast Reconstr Surg. 1985 Jun;75(6):785–790. doi: 10.1097/00006534-198506000-00001. [DOI] [PubMed] [Google Scholar]
- Horne R. S., Hurley J. V., Crowe D. M., Ritz M., O'Brien B. M., Arnold L. I. Wound healing in foetal sheep: a histological and electron microscope study. Br J Plast Surg. 1992 Jul;45(5):333–344. doi: 10.1016/0007-1226(92)90001-e. [DOI] [PubMed] [Google Scholar]
- Krummel T. M., Nelson J. M., Diegelmann R. F., Lindblad W. J., Salzberg A. M., Greenfield L. J., Cohen I. K. Fetal response to injury in the rabbit. J Pediatr Surg. 1987 Jul;22(7):640–644. doi: 10.1016/s0022-3468(87)80117-3. [DOI] [PubMed] [Google Scholar]
- Longaker M. T., Adzick N. S. The biology of fetal wound healing: a review. Plast Reconstr Surg. 1991 Apr;87(4):788–798. doi: 10.1097/00006534-199104000-00032. [DOI] [PubMed] [Google Scholar]
- Mast B. A., Diegelmann R. F., Krummel T. M., Cohen I. K. Scarless wound healing in the mammalian fetus. Surg Gynecol Obstet. 1992 May;174(5):441–451. [PubMed] [Google Scholar]
- Merkel J. R., DiPaolo B. R., Hallock G. G., Rice D. C. Type I and type III collagen content of healing wounds in fetal and adult rats. Proc Soc Exp Biol Med. 1988 Apr;187(4):493–497. doi: 10.3181/00379727-187-42694. [DOI] [PubMed] [Google Scholar]
- Robinson B. W., Goss A. N. Intra-uterine healing of fetal rat cheek wounds. Cleft Palate J. 1981 Oct;18(4):251–255. [PubMed] [Google Scholar]
- Rowsell A. R. The intra-uterine healing of foetal muscle wounds: experimental study in the rat. Br J Plast Surg. 1984 Oct;37(4):635–642. doi: 10.1016/0007-1226(84)90166-8. [DOI] [PubMed] [Google Scholar]
- Somasundaram K., Prathap K. Intra-uterine healing of skin wounds in rabbit foetuses. J Pathol. 1970 Feb;100(2):81–86. doi: 10.1002/path.1711000202. [DOI] [PubMed] [Google Scholar]
- Somasundaram K., Prathap K. The effect of exclusion of amniotic fluid on intra-uterine healing of skin wounds in rabbit foetuses. J Pathol. 1972 Jun;107(2):127–130. doi: 10.1002/path.1711070208. [DOI] [PubMed] [Google Scholar]
- Stegemann H., Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967 Nov;18(2):267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- Sykes B., Puddle B., Francis M., Smith R. The estimation of two collagens from human dermis by interrupted gel electrophoresis. Biochem Biophys Res Commun. 1976 Oct 18;72(4):1472–1480. doi: 10.1016/s0006-291x(76)80180-5. [DOI] [PubMed] [Google Scholar]
- Whitby D. J., Ferguson M. W. The extracellular matrix of lip wounds in fetal, neonatal and adult mice. Development. 1991 Jun;112(2):651–668. doi: 10.1242/dev.112.2.651. [DOI] [PubMed] [Google Scholar]