Abstract
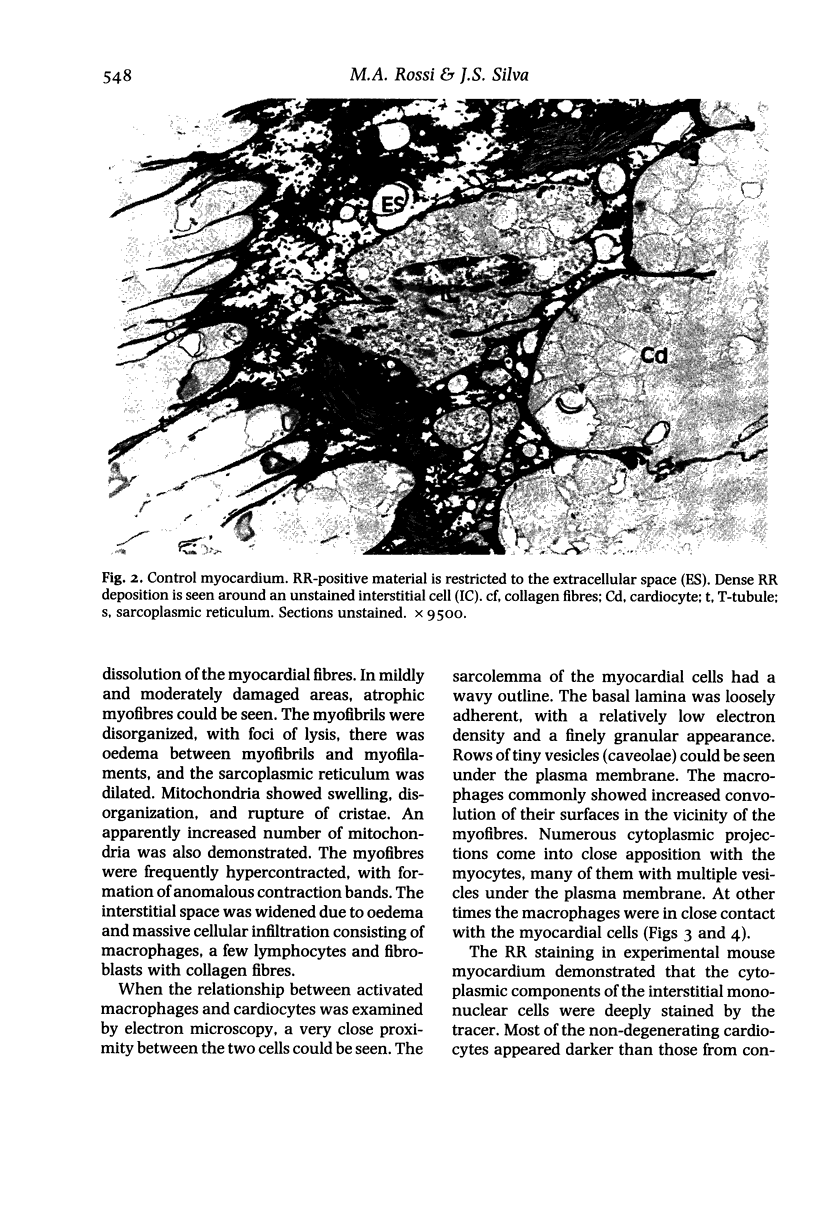
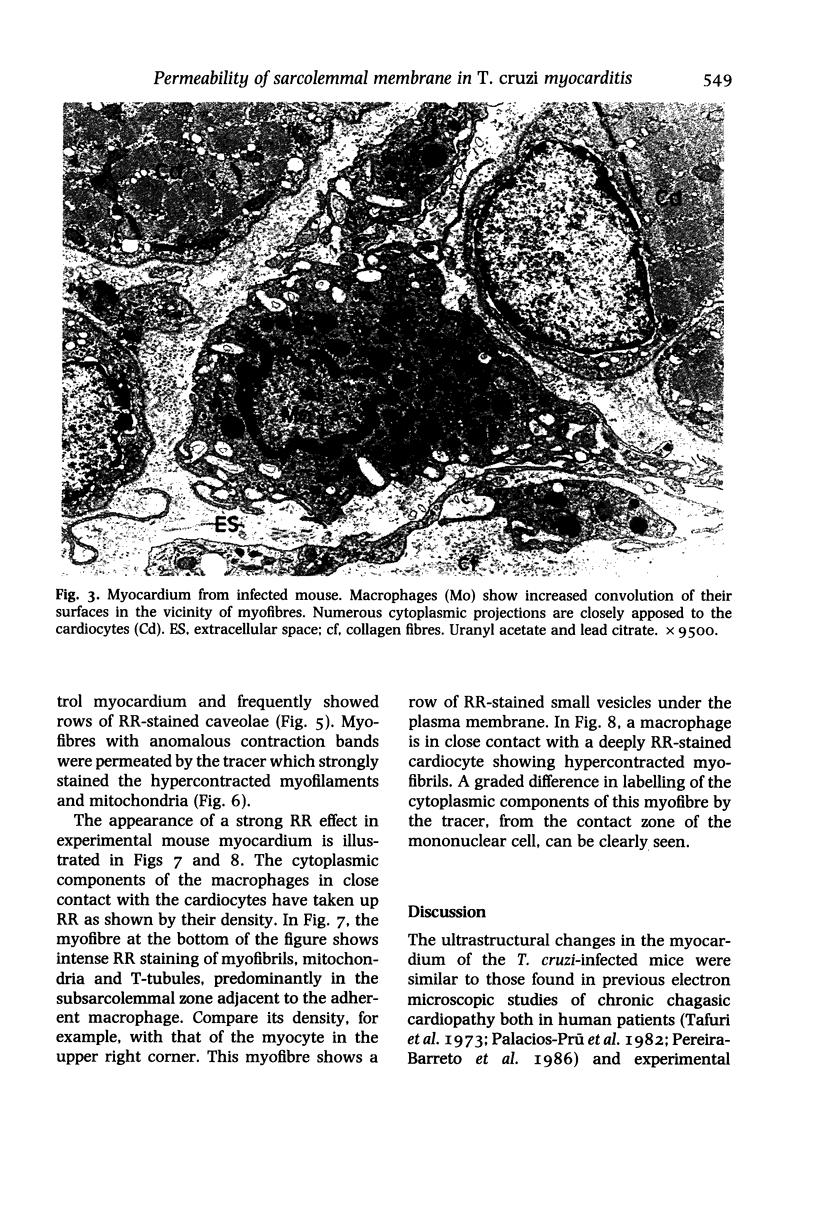
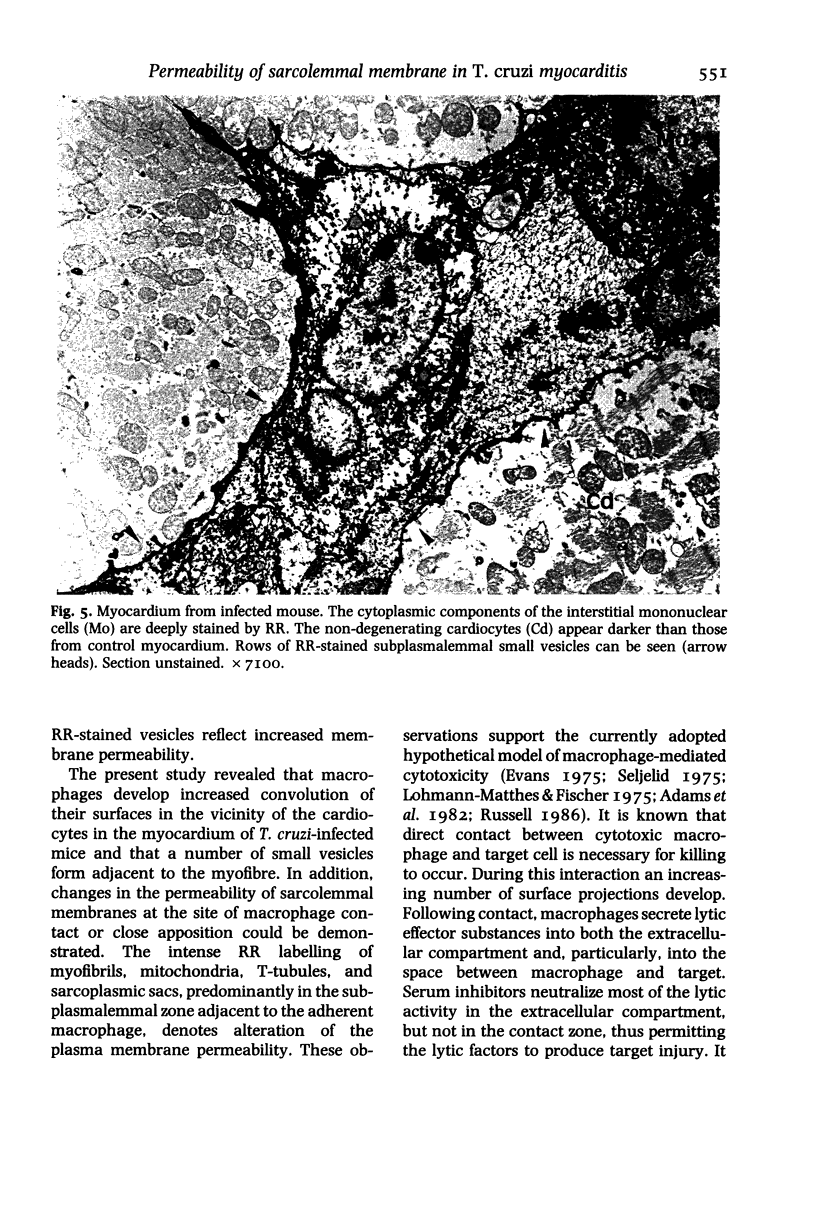
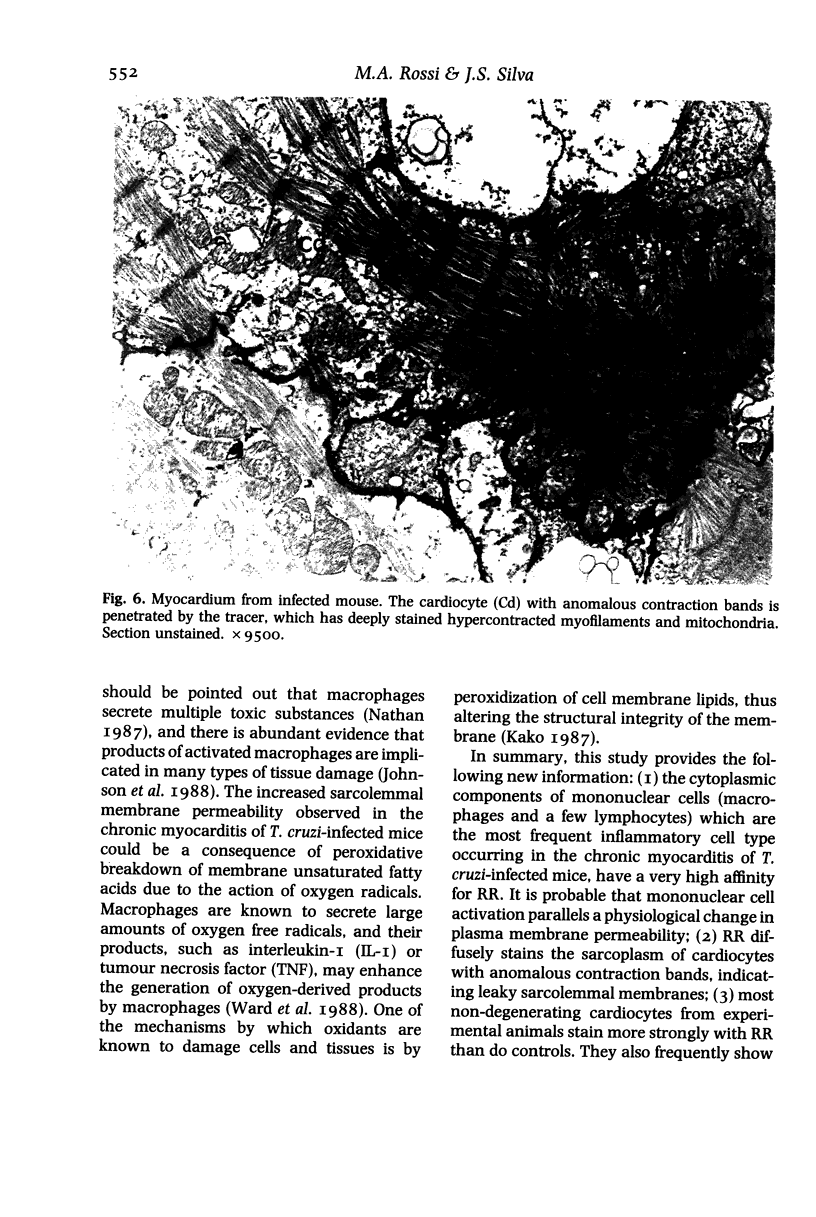
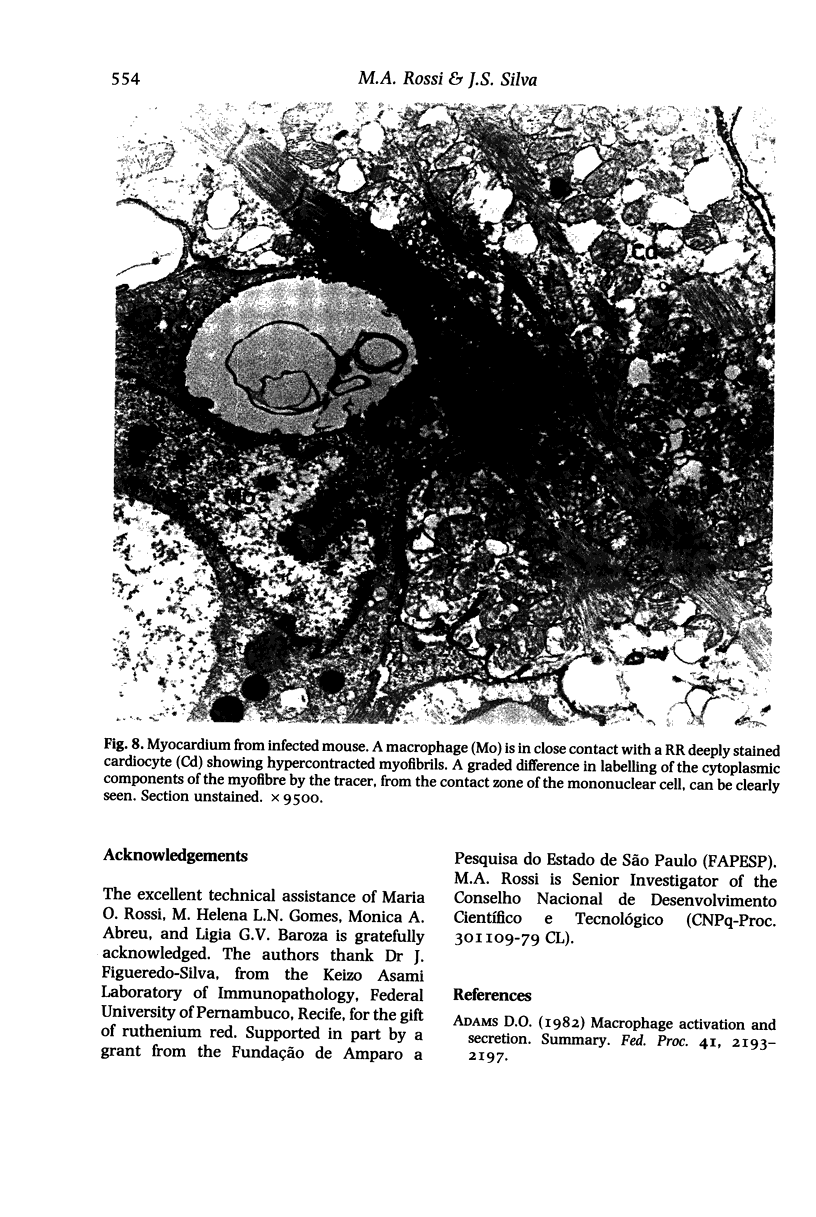
Sarcolemmal membrane permeability characteristics have been investigated, particularly at the site of macrophage contact, in experimental chronic Trypanosoma cruzi myocarditis in BALB/c mice, employing ruthenium red (RR) as an electron tracer. The ultrastructural features of the myocardium from infected animals were similar to those previously described. Briefly, focal myocarditis was detected, with areas of myocytolytic necrosis, atrophic myofibres, an inflammatory response composed of mononuclear cells, predominantly macrophages and a few lymphocytes, and interstitial fibrosis. This study provided the following new information: (I) the cytoplasmic components of mononuclear cells have a very high affinity for RR. It is conceivable that mononuclear cell activation parallels a physiological change in plasma membrane permeability; (2) RR diffusely strains the sarcoplasm of cardiocytes with anomalous contraction bands, indicating leaky sarcolemmal membranes; (3) most non-degenerating cardiocytes from experimental animals appear darker with RR staining than controls. They also frequently show rows of RR-stained sub-plasmalemmal tiny vesicles. Both changes probably reflect increased membrane permeability; (4) RR intensely labels the cytoplasmic components of cardiocytes at the site of macrophage contact or close apposition, indicating areas of altered membrane with remarkably increased permeability. This observation provides insight into a role for the macrophages in myocardial cell damage in experimental chronic Trypanosoma cruzi myocarditis. An obvious consequence of increased membrane permeability is that it may cause impairment of the transmembrane ion gradients and cause loss of intracellular elements, thus contributing to cardiocyte death. Furthermore, the findings in the present study imply that one of the consequences of macrophage-mediated cytotoxicity may be alteration in the permeability of the plasma membranes of nearby target cells, possibly due to a change in the structural integrity of the membrane resulting from peroxidation of cell membrane lipids.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Johnson W. J., Marino P. A. Mechanisms of target recognition and destruction in macrophage-mediated tumor cytotoxicity. Fed Proc. 1982 Apr;41(6):2212–2221. [PubMed] [Google Scholar]
- Andrade Z. A. Mechanisms of myocardial damage in Trypanosoma cruzi infection. Ciba Found Symp. 1983;99:214–233. doi: 10.1002/9780470720806.ch12. [DOI] [PubMed] [Google Scholar]
- Ganote C. E. Contraction band necrosis and irreversible myocardial injury. J Mol Cell Cardiol. 1983 Feb;15(2):67–73. doi: 10.1016/0022-2828(83)90283-3. [DOI] [PubMed] [Google Scholar]
- Kako K. J. Free radical effects on membrane protein in myocardial ischemia/reperfusion injury. J Mol Cell Cardiol. 1987 Feb;19(2):209–211. doi: 10.1016/s0022-2828(87)80563-1. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. II. Fine structural localization in animal tissues. Anat Rec. 1971 Nov;171(3):369–415. doi: 10.1002/ar.1091710303. [DOI] [PubMed] [Google Scholar]