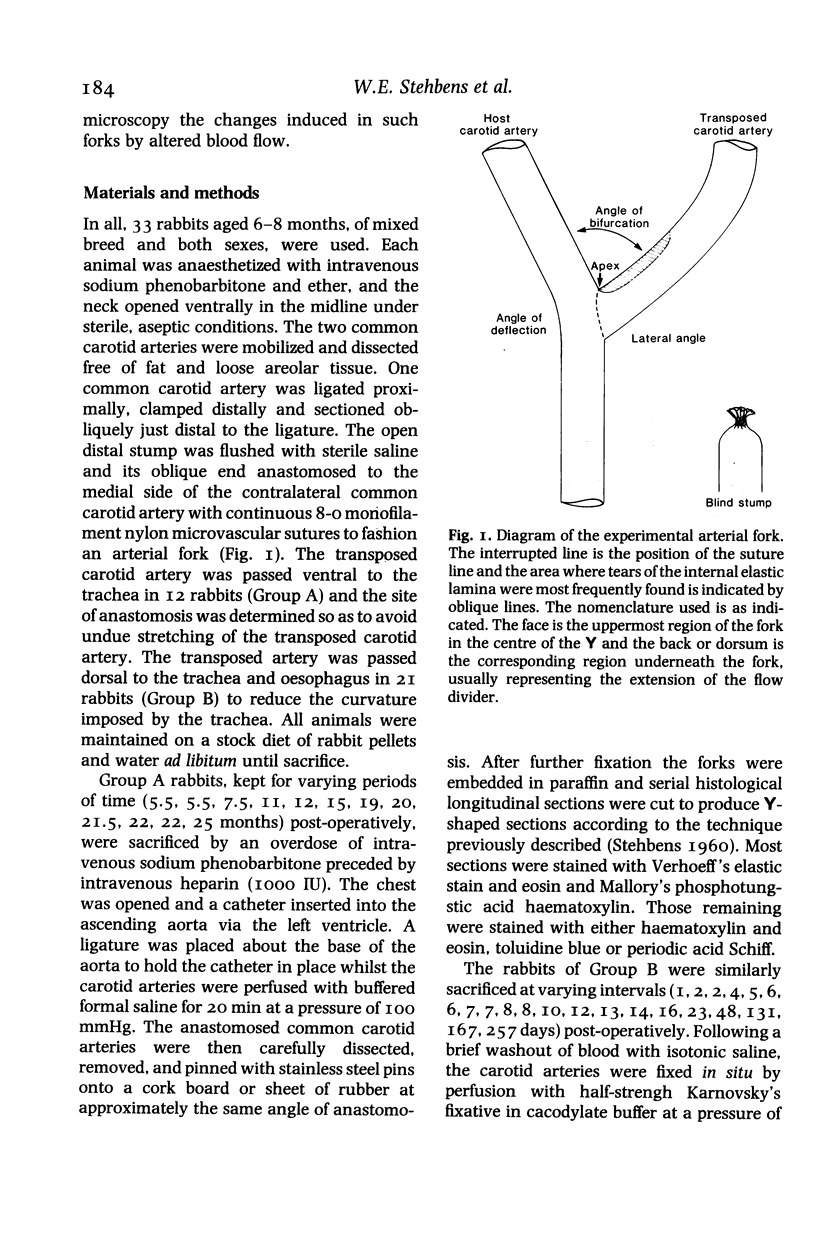
Abstract
Experimental arterial forks were fashioned by anastomosing one common carotid artery to the contralateral vessel by microvascular surgery in rabbits. In one rabbit group the forks were examined histologically by the serial section technique from 5.5 to 25 months postoperatively. The second group was used for scanning electron microscopy of the arterial endothelial surface from 1 to 257 days post-operatively. Intimal proliferation was observed at the lateral angles histologically at sites comparable to those where intimal proliferation occurs spontaneously in human infants. In addition, disruption of the internal elastic lamina with minimal intimal proliferation occurred at the sides of the neo-apex, mostly in the transposed artery. These disruptions corresponded to predominantly transversely orientated tears of the internal elastic lamina from 8 days post-operatively in the scanning electron microscopic study. They were similar to the early atrophic changes preceding berry aneurysm formation in human cerebral arterial forks. The results indicate that both intimal pads (cushions) and elastic tears can be haemodynamically induced.
Full text
PDF

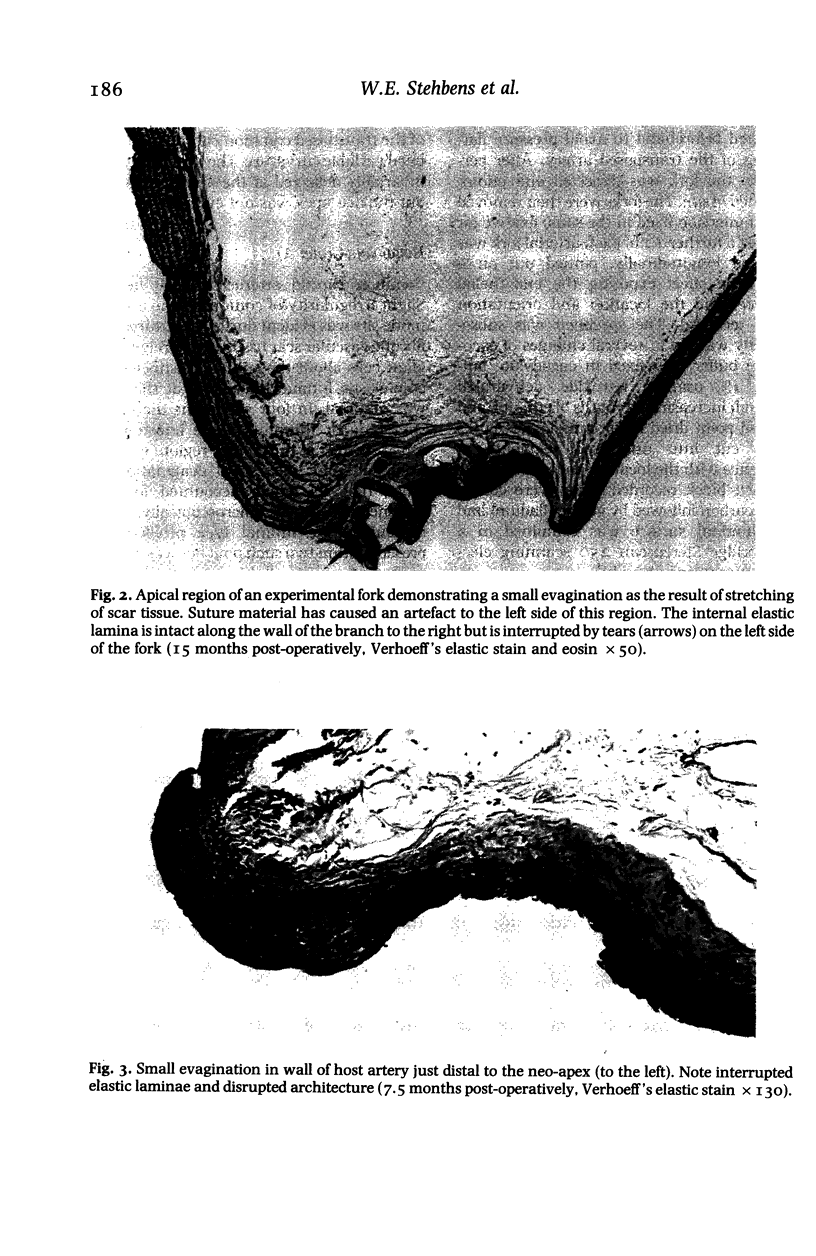

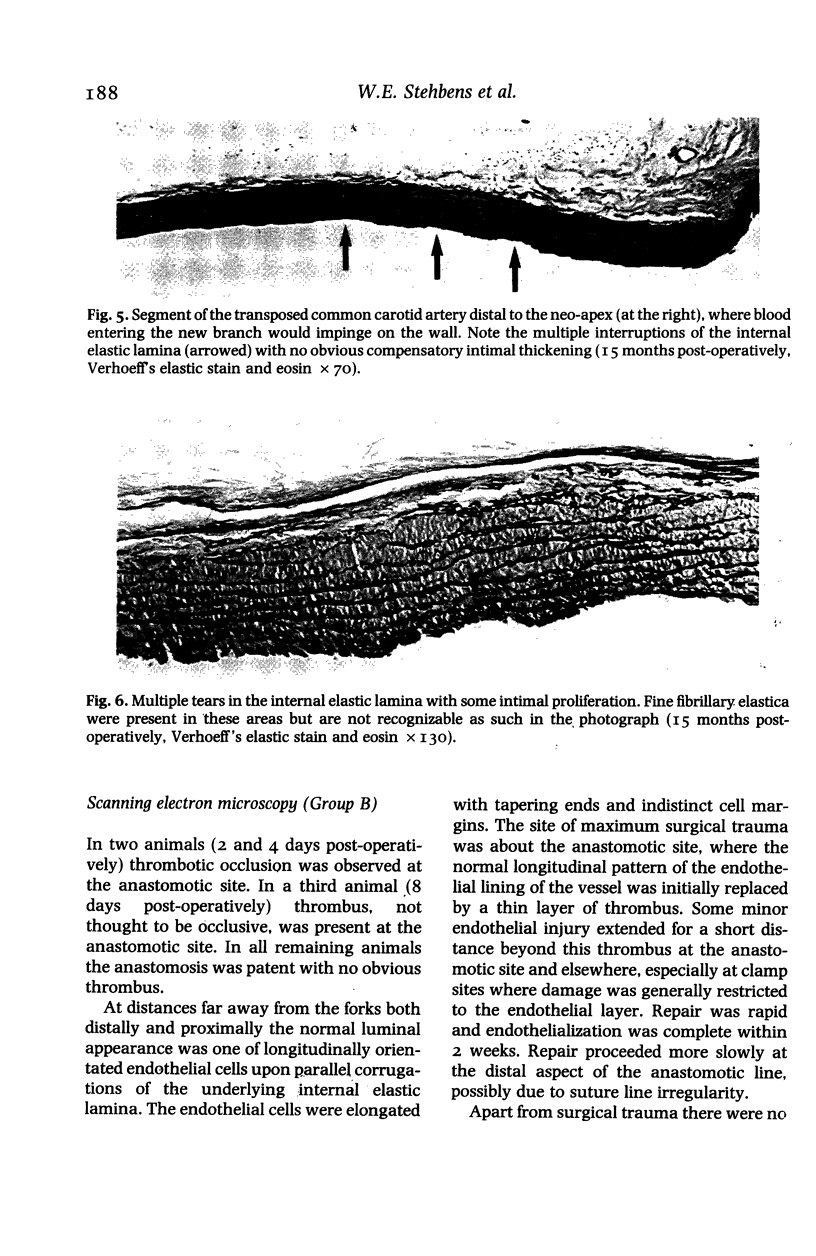
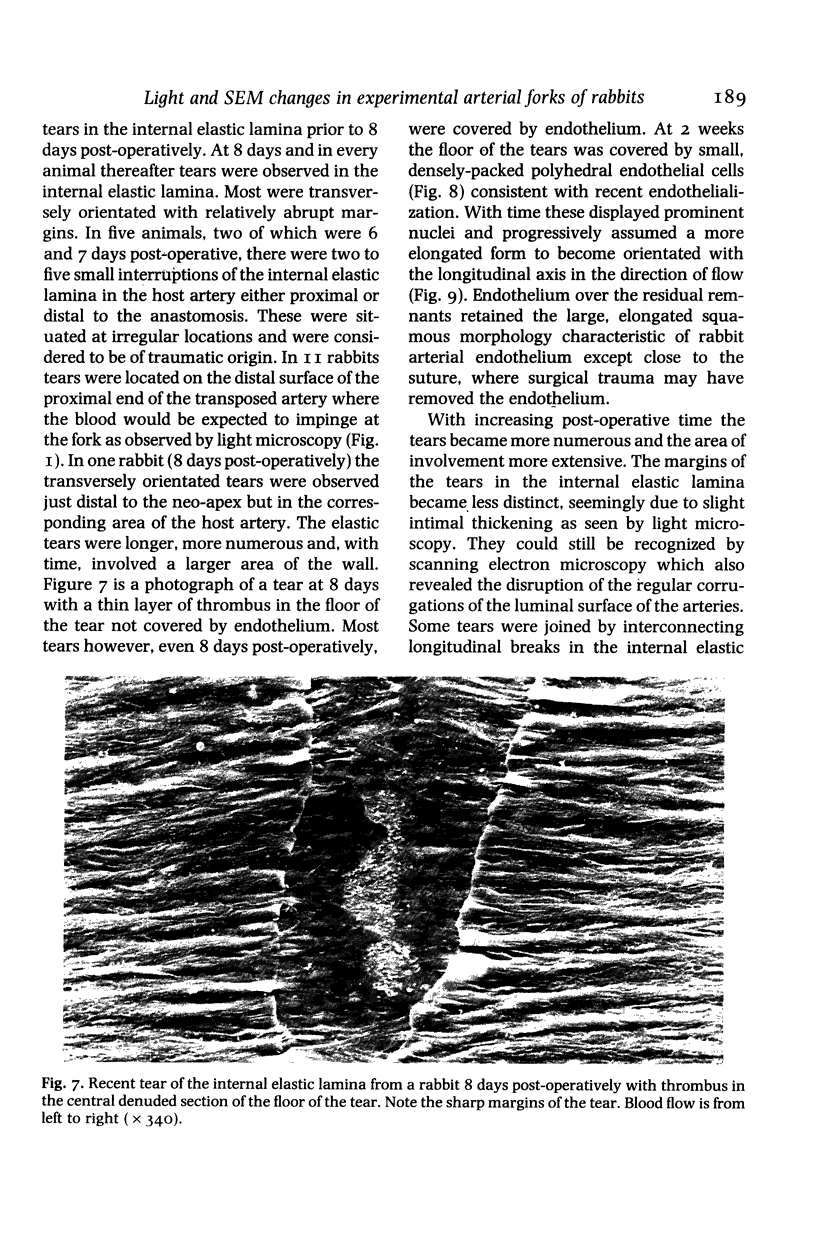




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Greenhill N. S., Stehbens W. E. Haemodynamically-induced intimal tears in experimental U-shaped arterial loops as seen by scanning electron microscopy. Br J Exp Pathol. 1985 Oct;66(5):577–584. [PMC free article] [PubMed] [Google Scholar]
- Greenhill N. S., Stehbens W. E. Scanning electron microscopic investigation of the afferent arteries of experimental femoral arteriovenous fistulae in rabbits. Pathology. 1987 Jan;19(1):22–27. doi: 10.3109/00313028709065130. [DOI] [PubMed] [Google Scholar]
- Hazama F., Hashimoto N. An animal model of cerebral aneurysms. Neuropathol Appl Neurobiol. 1987 Mar-Apr;13(2):77–90. doi: 10.1111/j.1365-2990.1987.tb00173.x. [DOI] [PubMed] [Google Scholar]
- Ishiharajima S., Aida T., Nakagawa R., Kameyama K., Sugano K., Oguro T., Asano G. Early membrane damage during ischemia in rat heart. Exp Mol Pathol. 1986 Feb;44(1):1–6. doi: 10.1016/0014-4800(86)90027-4. [DOI] [PubMed] [Google Scholar]
- Meyer W. W., Lind J. Calcifications of iliac arteries in newborns and infants. Arch Dis Child. 1972 Jun;47(253):364–372. doi: 10.1136/adc.47.253.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne-Pellegrin M. J., Weill D., Coutard M. A study of the influence of steroid hormones, hemodynamics, and environmental factors on the formation of small "spontaneous" lesions in the rat caudal artery. Lab Invest. 1980 Nov;43(5):471–479. [PubMed] [Google Scholar]
- STEHBENS W. E. Focal intimal proliferation in the cerebral arteries. Am J Pathol. 1960 Mar;36:289–301. [PMC free article] [PubMed] [Google Scholar]
- STEHBENS W. E. Histopathology of cerebral aneurysms. Arch Neurol. 1963 Mar;8:272–285. doi: 10.1001/archneur.1963.00460030056005. [DOI] [PubMed] [Google Scholar]
- Stehbens W. E. Changes in the cross-sectional area of the arterial fork. Angiology. 1974 Oct;25(9):561–575. doi: 10.1177/000331977402500902. [DOI] [PubMed] [Google Scholar]