Abstract
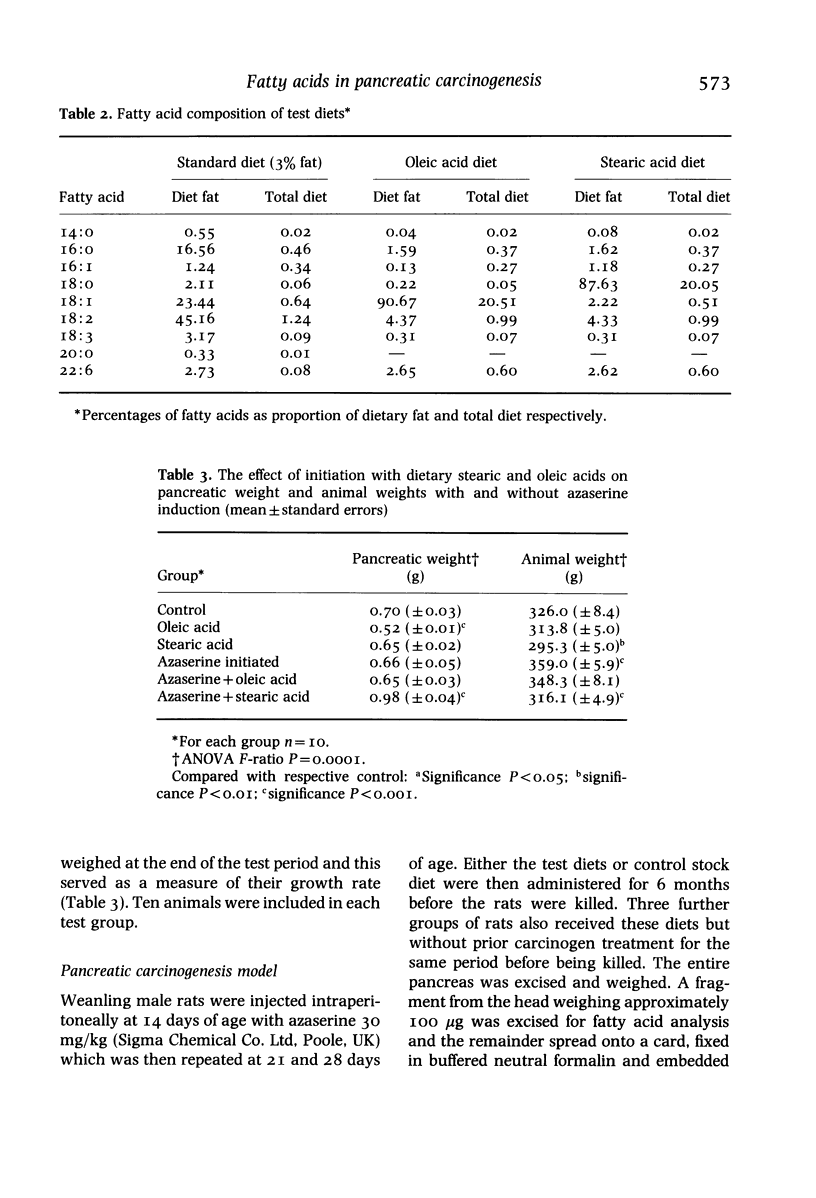
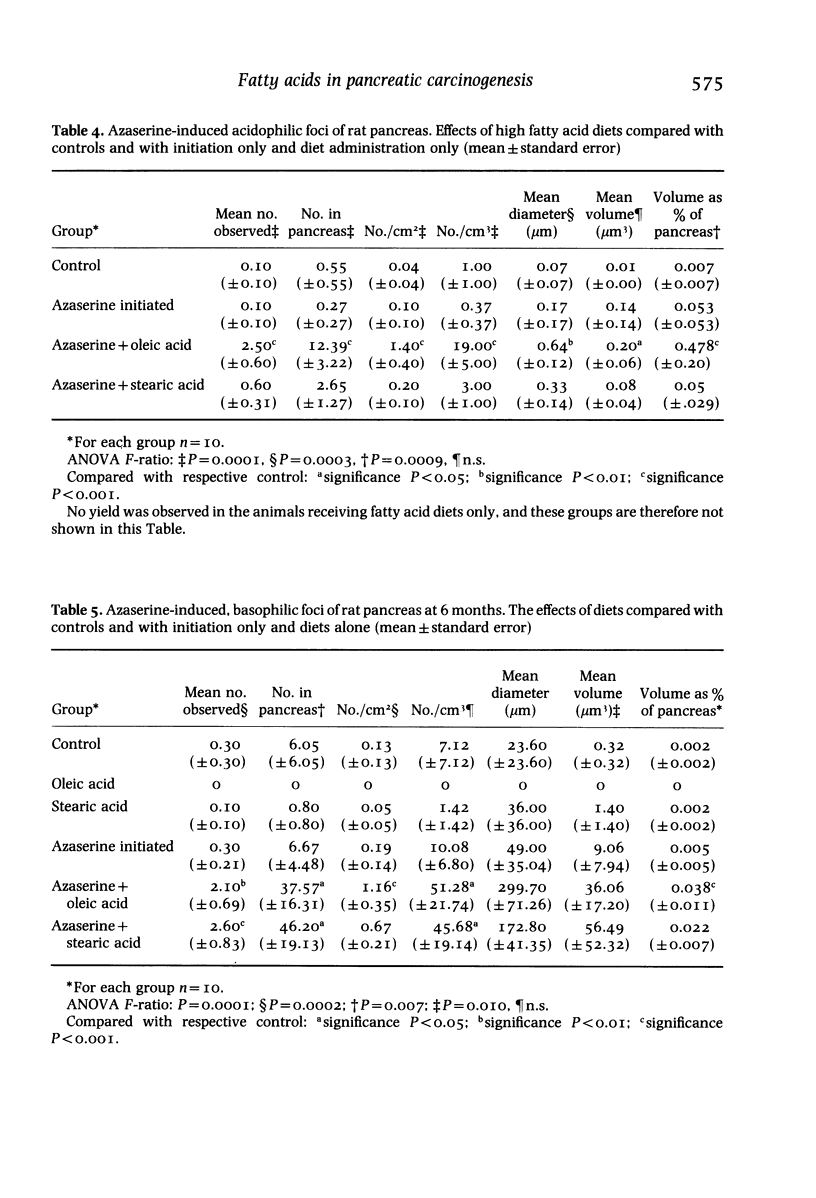
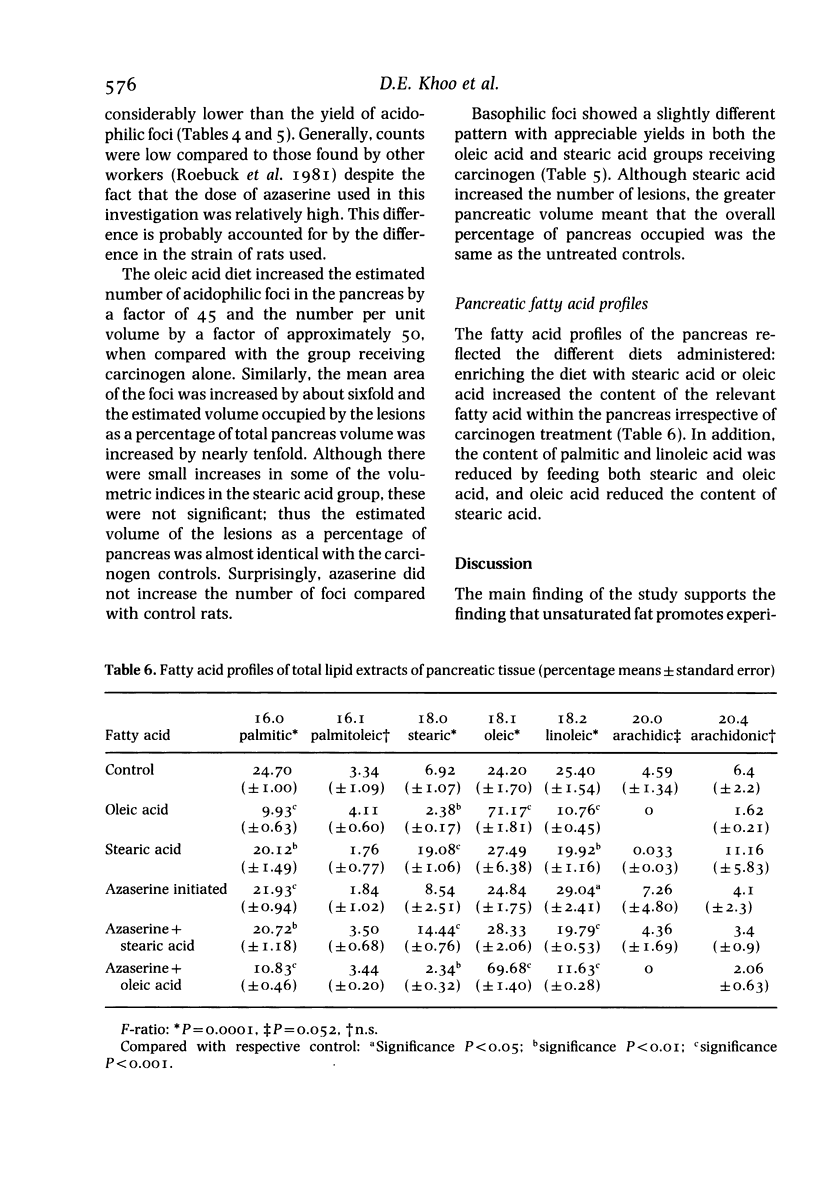
Diets enriched with fat, especially unsaturated fat, promote experimental pancreatic carcinogenesis, but little is known of the effects of individual fatty acids. The effect of stearic and oleic acid on pancreatic fatty acids and atypical acinar cell nodules (preneoplastic lesions) was studied in 14-day-old weanling male Leeds strain rats (n = 60) given the carcinogen azaserine. Rats were allocated to one of six groups: untreated controls (n = 10), 20% stearic acid diet (n = 10), 20% oleic acid diet (n = 10), carcinogen alone (n = 10), carcinogen plus 20% stearic acid diet (n = 10) or carcinogen plus 20% oleic acid diet (n = 10). Azaserine was administered by intraperitoneal injection in a dose of 30 mg/kg at 2, 3 and 4 weeks of age. When total lipid extracts of pancreas were examined, there was an increase in stearic acid in the stearic acid fed group and an increase in oleic acid in the oleic acid fed group, irrespective of carcinogen treatment. The relative content of all other pancreatic fatty acids was suppressed by feeding oleic acid. At 26 weeks, the number and volumetric indices of pancreatic atypical acinar cell nodules was increased only in rats given azaserine and oleic acid. The enhancing effect of oleic acid on pancreatic carcinogenesis may be associated with pancreatic fatty acid changes.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-el-Ela S. H., Prasse K. W., Carroll R., Wade A. E., Dharwadkar S., Bunce O. R. Eicosanoid synthesis in 7,12-dimethylbenz(a)anthracene-induced mammary carcinomas in Sprague-Dawley rats fed primrose oil, menhaden oil or corn oil diet. Lipids. 1988 Oct;23(10):948–954. doi: 10.1007/BF02536342. [DOI] [PubMed] [Google Scholar]
- Aylsworth C. F., Welsch C. W., Kabara J. J., Trosko J. E. Effects of fatty acids on gap junctional communication: possible role in tumor promotion by dietary fat. Lipids. 1987 Jun;22(6):445–454. doi: 10.1007/BF02537277. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay G. K., Imagawa W., Wallace D., Nandi S. Linoleate metabolites enhance the in vitro proliferative response of mouse mammary epithelial cells to epidermal growth factor. J Biol Chem. 1987 Feb 25;262(6):2750–2756. [PubMed] [Google Scholar]
- Bennett A. S. Effect of dietary stearic acid on the genesis of spontaneous mammary adenocarcinomas in strain A/ST mice. Int J Cancer. 1984 Oct 15;34(4):529–533. doi: 10.1002/ijc.2910340416. [DOI] [PubMed] [Google Scholar]
- Burns C. P., Spector A. A. Membrane fatty acid modification in tumor cells: a potential therapeutic adjunct. Lipids. 1987 Mar;22(3):178–184. doi: 10.1007/BF02537299. [DOI] [PubMed] [Google Scholar]
- Carroll K. K., Hopkins G. J., Kennedy T. G., Davidson M. B. Essential fatty acids in relation to mammary carcinogenesis. Prog Lipid Res. 1981;20:685–690. doi: 10.1016/0163-7827(81)90126-0. [DOI] [PubMed] [Google Scholar]
- Chan P. C., Ferguson K. A., Dao T. L. Effects of different dietary fats on mammary carcinogenesis. Cancer Res. 1983 Mar;43(3):1079–1083. [PubMed] [Google Scholar]
- Friedman E., Isaksson P., Rafter J., Marian B., Winawer S., Newmark H. Fecal diglycerides as selective endogenous mitogens for premalignant and malignant human colonic epithelial cells. Cancer Res. 1989 Feb 1;49(3):544–548. [PubMed] [Google Scholar]
- Gudjonsson B., Livstone E. M., Spiro H. M. Cancer of the pancreas: diagnostic accuracy and survival statistics. Cancer. 1978 Nov;42(5):2494–2506. doi: 10.1002/1097-0142(197811)42:5<2494::aid-cncr2820420554>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Karmali R. A., Marsh J., Fuchs C. Effect of omega-3 fatty acids on growth of a rat mammary tumor. J Natl Cancer Inst. 1984 Aug;73(2):457–461. doi: 10.1093/jnci/73.2.457. [DOI] [PubMed] [Google Scholar]
- Levin D. L., Connelly R. R., Devesa S. S. Demographic characteristics of cancer of the pancreas: mortality, incidence, and survival. Cancer. 1981 Mar 15;47(6 Suppl):1456–1468. doi: 10.1002/1097-0142(19810315)47:6+<1456::aid-cncr2820471404>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Longnecker D. S., Curphey T. J. Adenocarcinoma of the pancreas in azaserine-treated rats. Cancer Res. 1975 Aug;35(8):2249–2258. [PubMed] [Google Scholar]
- Longnecker D. S., Shinozuka H., Dekker A. Focal acinar cell dysplasia in human pancreas. Cancer. 1980 Feb;45(3):534–540. doi: 10.1002/1097-0142(19800201)45:3<534::aid-cncr2820450320>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Mack T. M., Paganini-Hill A. Epidemiology of pancreas cancer in Los Angeles. Cancer. 1981 Mar 15;47(6 Suppl):1474–1484. doi: 10.1002/1097-0142(19810315)47:6+<1474::aid-cncr2820471406>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Miller A. B., Kelly A., Choi N. W., Matthews V., Morgan R. W., Munan L., Burch J. D., Feather J., Howe G. R., Jain M. A study of diet and breast cancer. Am J Epidemiol. 1978 Jun;107(6):499–509. doi: 10.1093/oxfordjournals.aje.a112569. [DOI] [PubMed] [Google Scholar]
- Murakami K., Routtenberg A. Direct activation of purified protein kinase C by unsaturated fatty acids (oleate and arachidonate) in the absence of phospholipids and Ca2+. FEBS Lett. 1985 Nov 18;192(2):189–193. doi: 10.1016/0014-5793(85)80105-8. [DOI] [PubMed] [Google Scholar]
- Rao M. S., Upton M. P., Subbarao V., Scarpelli D. G. Two populations of cells with differing proliferative capacities in atypical acinar cell foci induced by 4-hydroxyaminoquinoline-1-oxide in the rat pancreas. Lab Invest. 1982 May;46(5):527–534. [PubMed] [Google Scholar]
- Reddy B. S., Maeura Y. Tumor promotion by dietary fat in azoxymethane-induced colon carcinogenesis in female F344 rats: influence of amount and source of dietary fat. J Natl Cancer Inst. 1984 Mar;72(3):745–750. [PubMed] [Google Scholar]
- Roebuck B. D., Baumgartner K. J., Thron C. D. Characterization of two populations of pancreatic atypical acinar cell foci induced by azaserine in the rat. Lab Invest. 1984 Feb;50(2):141–146. [PubMed] [Google Scholar]
- Roebuck B. D. Effects of high levels of dietary fats on the growth of azaserine-induced foci in the rat pancreas. Lipids. 1986 Apr;21(4):281–284. doi: 10.1007/BF02536413. [DOI] [PubMed] [Google Scholar]
- Roebuck B. D., Yager J. D., Jr, Longnecker D. S., Wilpone S. A. Promotion by unsaturated fat of azaserine-induced pancreatic carcinogenesis in the rat. Cancer Res. 1981 Oct;41(10):3961–3966. [PubMed] [Google Scholar]
- Sandermann H., Jr Regulation of membrane enzymes by lipids. Biochim Biophys Acta. 1978 Sep 29;515(3):209–237. doi: 10.1016/0304-4157(78)90015-1. [DOI] [PubMed] [Google Scholar]
- Tinsley I. J., Schmitz J. A., Pierce D. A. Influence of dietary fatty acids on the incidence of mammary tumors in the C3H mouse. Cancer Res. 1981 Apr;41(4):1460–1465. [PubMed] [Google Scholar]
- Wicha M. S., Liotta L. A., Kidwell W. R. Effects of free fatty acids on the growth of normal and neoplastic rat mammary epithelial cells. Cancer Res. 1979 Feb;39(2 Pt 1):426–435. [PubMed] [Google Scholar]
- Wynder E. L. An epidemiological evaluation of the causes of cancer of the pancreas. Cancer Res. 1975 Aug;35(8):2228–2233. [PubMed] [Google Scholar]
- Wynder E. L., Mabuchi K., Maruchi N., Fortner J. G. Epidemiology of cancer of the pancreas. J Natl Cancer Inst. 1973 Mar;50(3):645–667. doi: 10.1093/jnci/50.3.645. [DOI] [PubMed] [Google Scholar]


