Abstract
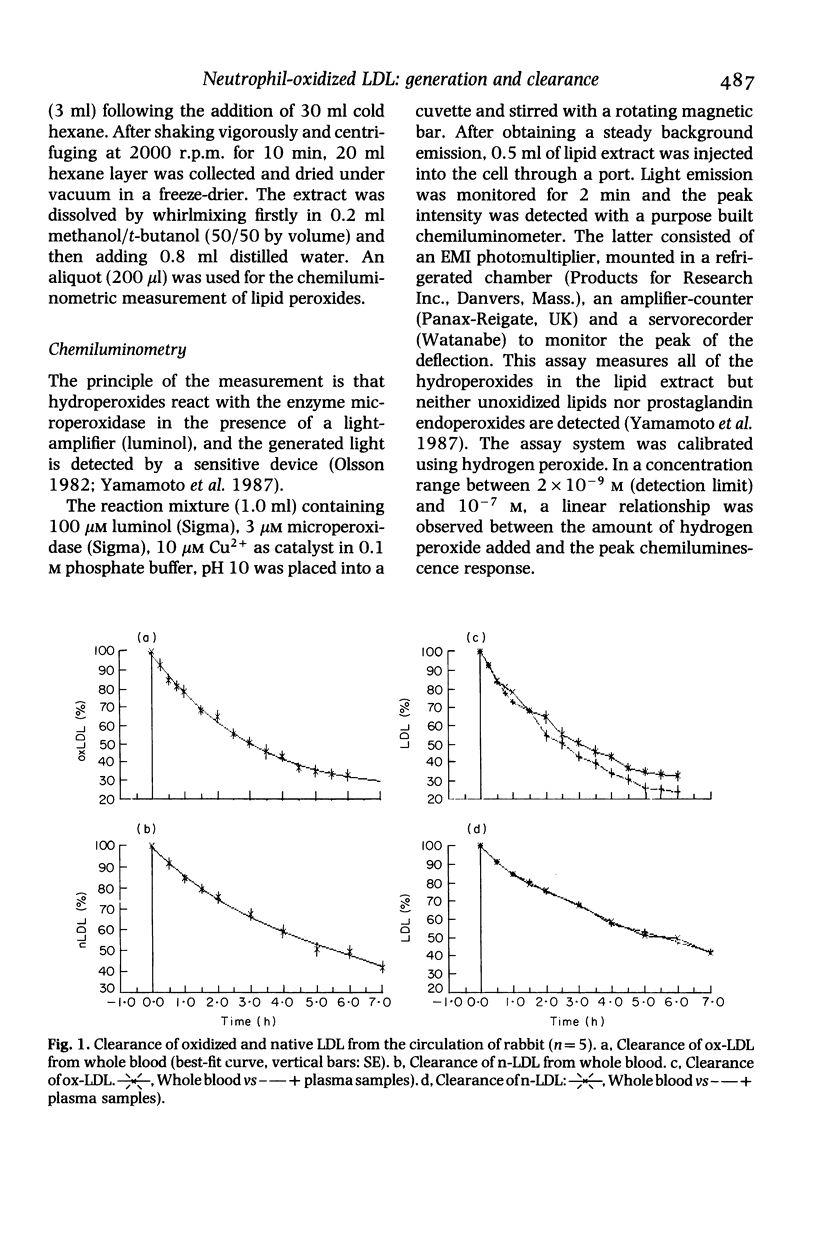
The prevailing concept of an extremely rapid disappearance of 'modified' low density lipoprotein (LDL) from the circulation was reinvestigated. Rabbit LDL was 'modified' by homologous activated (phagocytosing) polymorphonuclear leucocytes (PMLN), radiolabelled with a non-degradable ligand (125I-TC-LDL) and injected into rabbits. The plasma half-lives of 'modified' and native LDL were T1/2 = 2.5 and 5.75 h, respectively. Furthermore, the possibility of LDL oxidation in plasma by stimulated PMNL was investigated. Hirudin-anticoagulated human plasma was incubated with unstimulated or stimulated autologous PMNL. Chemiluminometry (reactants with microperoxidase) of the lipid extract of plasma after incubation showed lipid peroxidation to be induced by phagocytosing, but not by quiescent, leucocytes. These findings show that in plasma, stimulated leucocytes can 'modify' LDL and the circulatory half-life of the latter enables its contribution to atherogenesis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviram M., Dankner G., Brook J. G. Platelet secretory products increase low density lipoprotein oxidation, enhance its uptake by macrophages, and reduce its fluidity. Arteriosclerosis. 1990 Jul-Aug;10(4):559–563. doi: 10.1161/01.atv.10.4.559. [DOI] [PubMed] [Google Scholar]
- Berliner J. A., Territo M. C., Sevanian A., Ramin S., Kim J. A., Bamshad B., Esterson M., Fogelman A. M. Minimally modified low density lipoprotein stimulates monocyte endothelial interactions. J Clin Invest. 1990 Apr;85(4):1260–1266. doi: 10.1172/JCI114562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathcart M. K., Morel D. W., Chisolm G. M., 3rd Monocytes and neutrophils oxidize low density lipoprotein making it cytotoxic. J Leukoc Biol. 1985 Aug;38(2):341–350. doi: 10.1002/jlb.38.2.341. [DOI] [PubMed] [Google Scholar]
- Davies S. W., Ranjadayalan K., Wickens D. G., Dormandy T. L., Timmis A. D. Lipid peroxidation associated with successful thrombolysis. Lancet. 1990 Mar 31;335(8692):741–743. doi: 10.1016/0140-6736(90)90866-4. [DOI] [PubMed] [Google Scholar]
- Frei B., Stocker R., Ames B. N. Antioxidant defenses and lipid peroxidation in human blood plasma. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9748–9752. doi: 10.1073/pnas.85.24.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görög P. Activation of human blood monocytes by oxidized polyunsaturated fatty acids: a possible mechanism for the generation of lipid peroxides in the circulation. Int J Exp Pathol. 1991 Apr;72(2):227–237. [PMC free article] [PubMed] [Google Scholar]
- Görög P., Kakkar V. V. Increased uptake of monocyte-treated low density lipoproteins by aortic endothelium in vivo. Atherosclerosis. 1987 May;65(1-2):99–107. doi: 10.1016/0021-9150(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Görög P., Pearson J. D., Kakkar V. V. Generation of reactive oxygen metabolites by phagocytosing endothelial cells. Atherosclerosis. 1988 Jul;72(1):19–27. doi: 10.1016/0021-9150(88)90058-5. [DOI] [PubMed] [Google Scholar]
- Hiramatsu K., Rosen H., Heinecke J. W., Wolfbauer G., Chait A. Superoxide initiates oxidation of low density lipoprotein by human monocytes. Arteriosclerosis. 1987 Jan-Feb;7(1):55–60. doi: 10.1161/01.atv.7.1.55. [DOI] [PubMed] [Google Scholar]
- Jessup W., Darley-Usmar V., O'Leary V., Bedwell S. 5-Lipoxygenase is not essential in macrophage-mediated oxidation of low-density lipoprotein. Biochem J. 1991 Aug 15;278(Pt 1):163–169. doi: 10.1042/bj2780163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughton C. W., Ruddle D. L., Bedord C. J., Alderman E. L. Sera containing elevated nonesterified fatty acids from patients with angiographically documented coronary atherosclerosis cause marked lipid accumulation in cultured human arterial smooth muscle-derived cells. Atherosclerosis. 1988 Apr;70(3):233–246. doi: 10.1016/0021-9150(88)90174-8. [DOI] [PubMed] [Google Scholar]
- Palinski W., Rosenfeld M. E., Ylä-Herttuala S., Gurtner G. C., Socher S. S., Butler S. W., Parthasarathy S., Carew T. E., Steinberg D., Witztum J. L. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman R. C., Carew T. E., Glass C. K., Green S. R., Taylor C. A., Jr, Attie A. D. A radioiodinated, intracellularly trapped ligand for determining the sites of plasma protein degradation in vivo. Biochem J. 1983 Jun 15;212(3):791–800. doi: 10.1042/bj2120791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricevuti G., Mazzone A., De Servi S., Specchia G., Fratino P. New trends in coronary artery disease: the role of granulocyte activation. Atherosclerosis. 1989 Aug;78(2-3):261–265. doi: 10.1016/0021-9150(89)90232-3. [DOI] [PubMed] [Google Scholar]
- Rosen G. M., Freeman B. A. Detection of superoxide generated by endothelial cells. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7269–7273. doi: 10.1073/pnas.81.23.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M. E., Palinski W., Ylä-Herttuala S., Butler S., Witztum J. L. Distribution of oxidation specific lipid-protein adducts and apolipoprotein B in atherosclerotic lesions of varying severity from WHHL rabbits. Arteriosclerosis. 1990 May-Jun;10(3):336–349. doi: 10.1161/01.atv.10.3.336. [DOI] [PubMed] [Google Scholar]
- St Clair R. W., Greenspan P., Leight M. Enhanced cholesterol delivery to cells in culture by low density lipoproteins from hypercholesterolemic monkeys. Arteriosclerosis. 1983 Jan-Feb;3(1):77–86. doi: 10.1161/01.atv.3.1.77. [DOI] [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Stringer M. D., Görög P. G., Freeman A., Kakkar V. V. Lipid peroxides and atherosclerosis. BMJ. 1989 Feb 4;298(6669):281–284. doi: 10.1136/bmj.298.6669.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szamosi T., Gara I., Venekei I., Jávor A., Ceskel R., Knoll J. Serum lipids, lipid peroxides and the care of children with high risk atherosclerotic family history. Atherosclerosis. 1987 Nov;68(1-2):111–115. doi: 10.1016/0021-9150(87)90100-6. [DOI] [PubMed] [Google Scholar]
- Uysal M., Bulur H., Sener D., Oz H. Lipid peroxidation in patients with essential hypertension. Int J Clin Pharmacol Ther Toxicol. 1986 Sep;24(9):474–476. [PubMed] [Google Scholar]
- Wissler R. W. Update on the pathogenesis of atherosclerosis. Am J Med. 1991 Jul 31;91(1B):3S–9S. doi: 10.1016/0002-9343(91)90050-8. [DOI] [PubMed] [Google Scholar]
- Witztum J. L., Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991 Dec;88(6):1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Brodsky M. H., Baker J. C., Ames B. N. Detection and characterization of lipid hydroperoxides at picomole levels by high-performance liquid chromatography. Anal Biochem. 1987 Jan;160(1):7–13. doi: 10.1016/0003-2697(87)90606-3. [DOI] [PubMed] [Google Scholar]
- Zhang H. F., Basra H. J., Steinbrecher U. P. Effects of oxidatively modified LDL on cholesterol esterification in cultured macrophages. J Lipid Res. 1990 Aug;31(8):1361–1369. [PubMed] [Google Scholar]
- van Hinsbergh V. W., Scheffer M., Havekes L., Kempen H. J. Role of endothelial cells and their products in the modification of low-density lipoproteins. Biochim Biophys Acta. 1986 Aug 14;878(1):49–64. doi: 10.1016/0005-2760(86)90343-7. [DOI] [PubMed] [Google Scholar]


