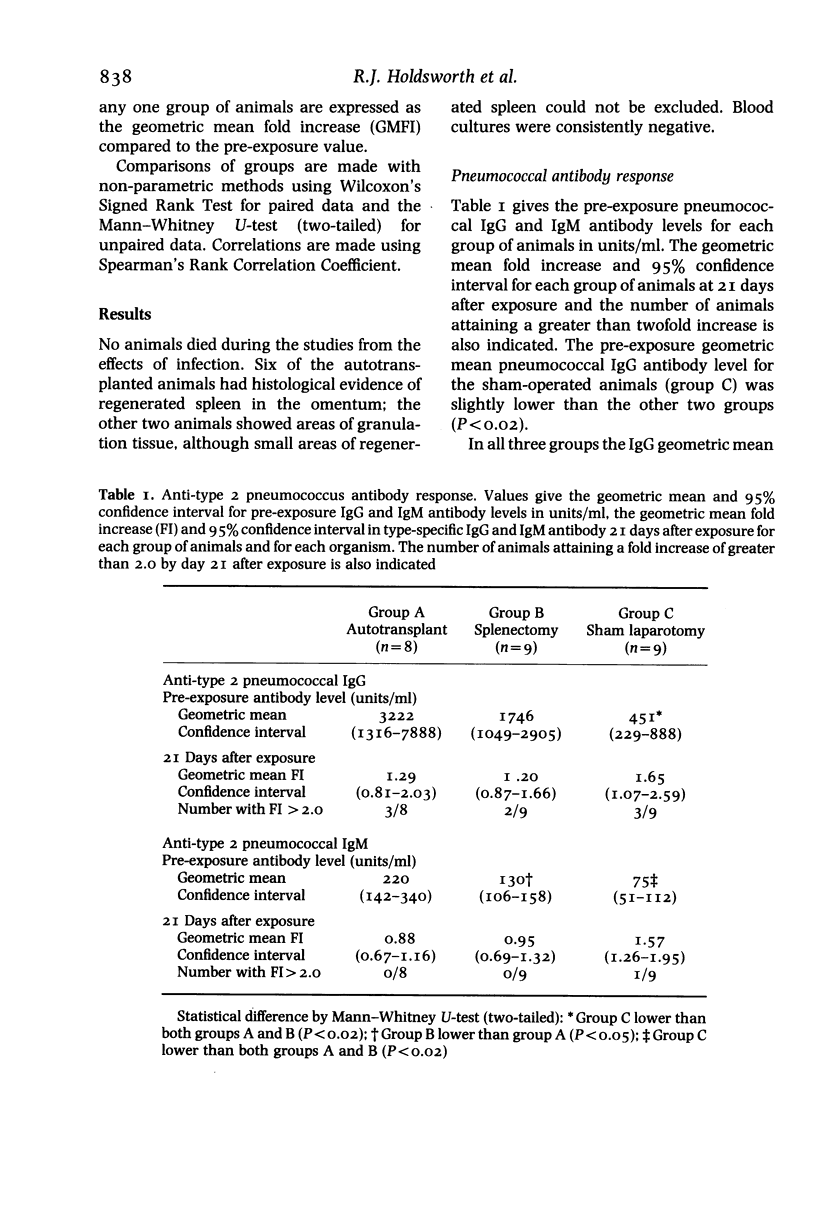
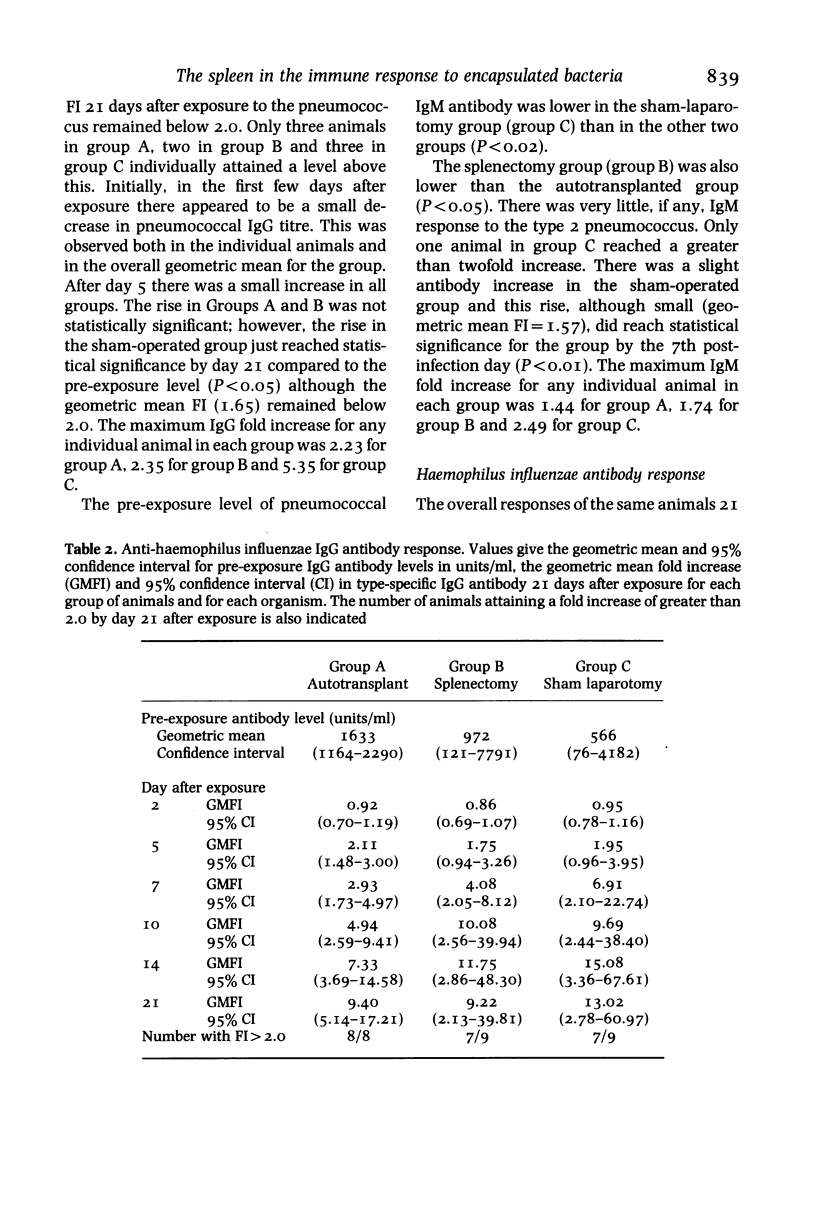
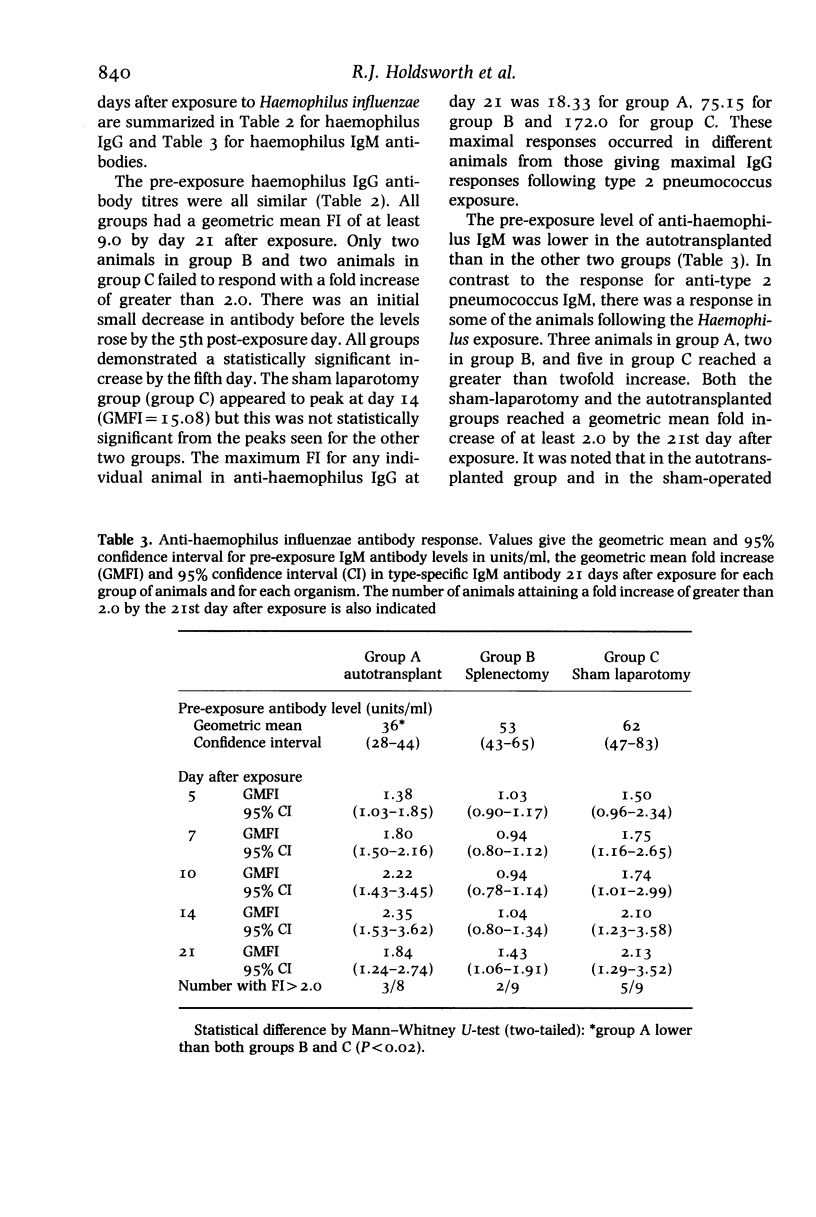
Abstract
Female New Zealand White Rabbits following splenectomy (n = 9), splenectomy with 50% splenic autotransplantation (n = 8) and sham laparotomy (n = 9) have been serially exposed to type 2 Streptococcus pneumoniae and Haemophilus influenzae by aerosol inhalation. Animals were sampled for 3 weeks after exposure and the IgG and IgM type-specific antibody response measured by enzyme-linked immunosorbent assay. Haemophilus influenzae initiated a substantial anti-haemophilus IgG response which was not diminished by splenectomy. The anti-haemophilus IgM response was present in sham-operated animals, absent following splenectomy, and partially restored by splenic autotransplantation. Type 2 Streptococcus pneumoniae induced a minimal IgG and IgM antibody response in all animals irrespective of the presence or absence of a spleen. The results support the role of the spleen in mediating IgM production against polysaccharide encapsulated bacteria. The differential degree of immune response produced by the two organisms may explain in part the differential frequency with which these two organisms infect man following splenectomy.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen V., Cohn J., Sorensen S. F. Immunological studies in children before and after splenectomy. Acta Paediatr Scand. 1976 Jul;65(4):409–415. doi: 10.1111/j.1651-2227.1976.tb04907.x. [DOI] [PubMed] [Google Scholar]
- Berglund G., Sannerstedt R., Andersson O., Wedel H., Wilhelmsen L., Hansson L., Sivertsson R., Wikstrand J. Coronary heart-disease after treatment of hypertension. Lancet. 1978 Jan 7;1(8054):1–5. doi: 10.1016/s0140-6736(78)90356-2. [DOI] [PubMed] [Google Scholar]
- Bohnsack J. F., Brown E. J. The role of the spleen in resistance to infection. Annu Rev Med. 1986;37:49–59. doi: 10.1146/annurev.me.37.020186.000405. [DOI] [PubMed] [Google Scholar]
- Brown E. J., Hosea S. W., Hammer C. H., Burch C. G., Frank M. M. A quantitative analysis of the interactions of antipneumococcal antibody and complement in experimental pneumococcal bacteremia. J Clin Invest. 1982 Jan;69(1):85–98. doi: 10.1172/JCI110444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. F., Van M., Abramson S. L., Fox E. J., Rich R. R. Cellular requirements for induction of human primary proliferative responses to trinitrophenyl-modified cells. J Immunol. 1984 Jan;132(1):19–24. [PubMed] [Google Scholar]
- Cohn D. A., Schiffman G. Immunoregulatory role of the spleen in antibody responses to pneumococcal polysaccharide antigens. Infect Immun. 1987 Jun;55(6):1375–1380. doi: 10.1128/iai.55.6.1375-1380.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cataldo A., Puleo S., Li Destri G., Racalbuto A., Trombatore G., Latteri F., Rodolico G. Splenic trauma and overwhelming postsplenectomy infection. Br J Surg. 1987 May;74(5):343–345. doi: 10.1002/bjs.1800740504. [DOI] [PubMed] [Google Scholar]
- Gavrilis P., Rothenberg S. P., Guy R. Correlation of low serum IgM levels with absence of functional splenic tissue in sickle cell disease syndromes. Am J Med. 1974 Oct;57(4):542–545. doi: 10.1016/0002-9343(74)90004-7. [DOI] [PubMed] [Google Scholar]
- Gray D., Chassoux D., MacLennan I. C., Bazin H. Selective depression of thymus-independent anti-DNP antibody responses induced by adult but not neonatal splenectomy. Clin Exp Immunol. 1985 Apr;60(1):78–86. [PMC free article] [PubMed] [Google Scholar]
- Linné T., Eriksson M., Lännergren K., Tordai P., Czar-Weidhagen B., Swedberg K. Splenic function after nonsurgical management of splenic rupture. J Pediatr. 1984 Aug;105(2):263–265. doi: 10.1016/s0022-3476(84)80125-0. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Zitron I. M., Mond J. J., Ahmed A., Scher I., Paul W. E. Surface immunoglobulin D as a functional receptor for a subclass of B lymphocytes. Immunol Rev. 1977;37:89–104. doi: 10.1111/j.1600-065x.1977.tb00246.x. [DOI] [PubMed] [Google Scholar]
- Oldfield S., Jenkins S., Yeoman H., Gray D., MacLennan I. C. Class and subclass anti-pneumococcal antibody responses in splenectomized patients. Clin Exp Immunol. 1985 Sep;61(3):664–673. [PMC free article] [PubMed] [Google Scholar]
- Pearson H. A. Splenectomy: its risks and its roles. Hosp Pract. 1980 Aug;15(8):85-9, 92-4. doi: 10.1080/21548331.1980.11946646. [DOI] [PubMed] [Google Scholar]
- Pedersen F. K., Henrichsen J., Schiffman G. Antibody response to vaccination with pneumococcal capsular polysaccharides in splenectomized children. Acta Paediatr Scand. 1982 May;71(3):451–455. doi: 10.1111/j.1651-2227.1982.tb09451.x. [DOI] [PubMed] [Google Scholar]
- Siber G. R., Schur P. H., Aisenberg A. C., Weitzman S. A., Schiffman G. Correlation between serum IgG-2 concentrations and the antibody response to bacterial polysaccharide antigens. N Engl J Med. 1980 Jul 24;303(4):178–182. doi: 10.1056/NEJM198007243030402. [DOI] [PubMed] [Google Scholar]
- Spirer Z. The role of the spleen in immunity and infection. Adv Pediatr. 1980;27:55–88. [PubMed] [Google Scholar]
- Van Wyck D. B., Witte M. H., Witte C. L., Strunk R. C. Humoral immunity in experimental hyposplenism. Surgery. 1978 Jul;84(1):134–139. [PubMed] [Google Scholar]


