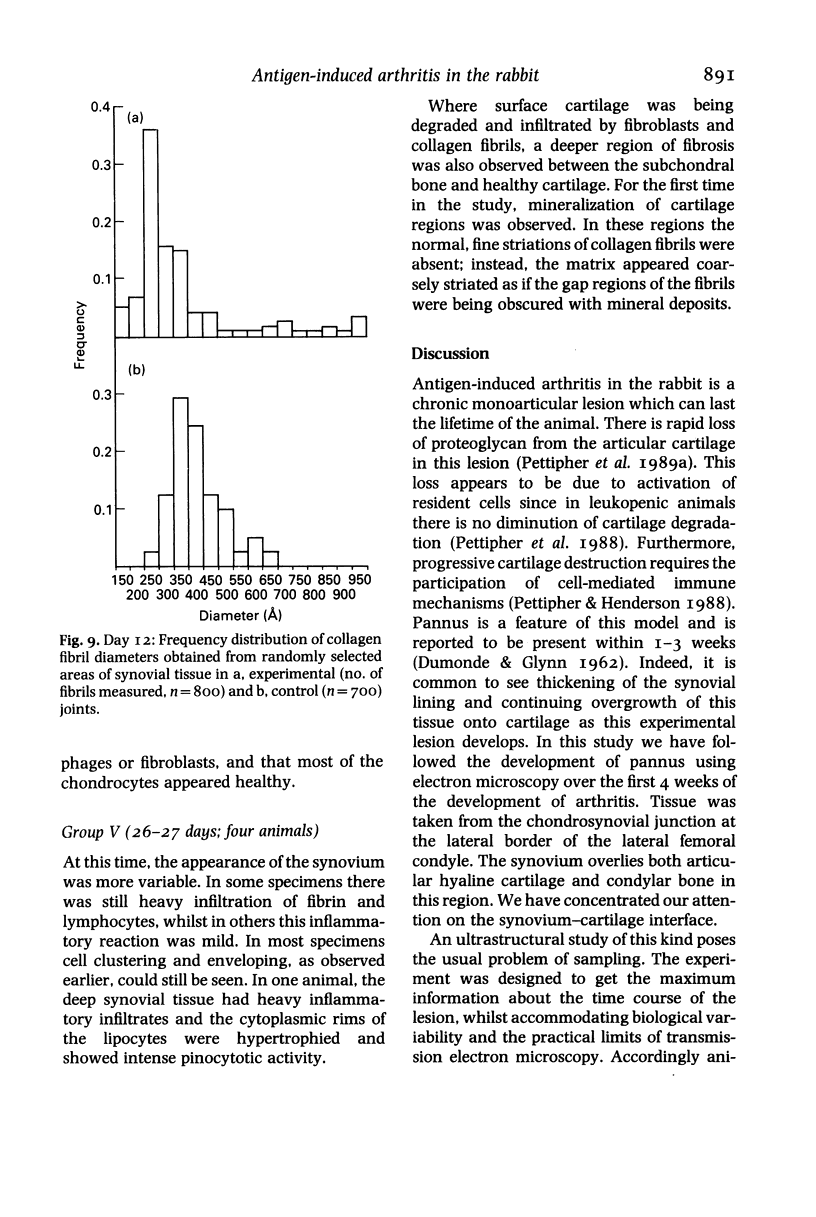
Abstract
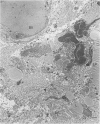
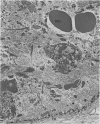
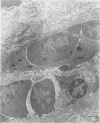
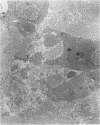
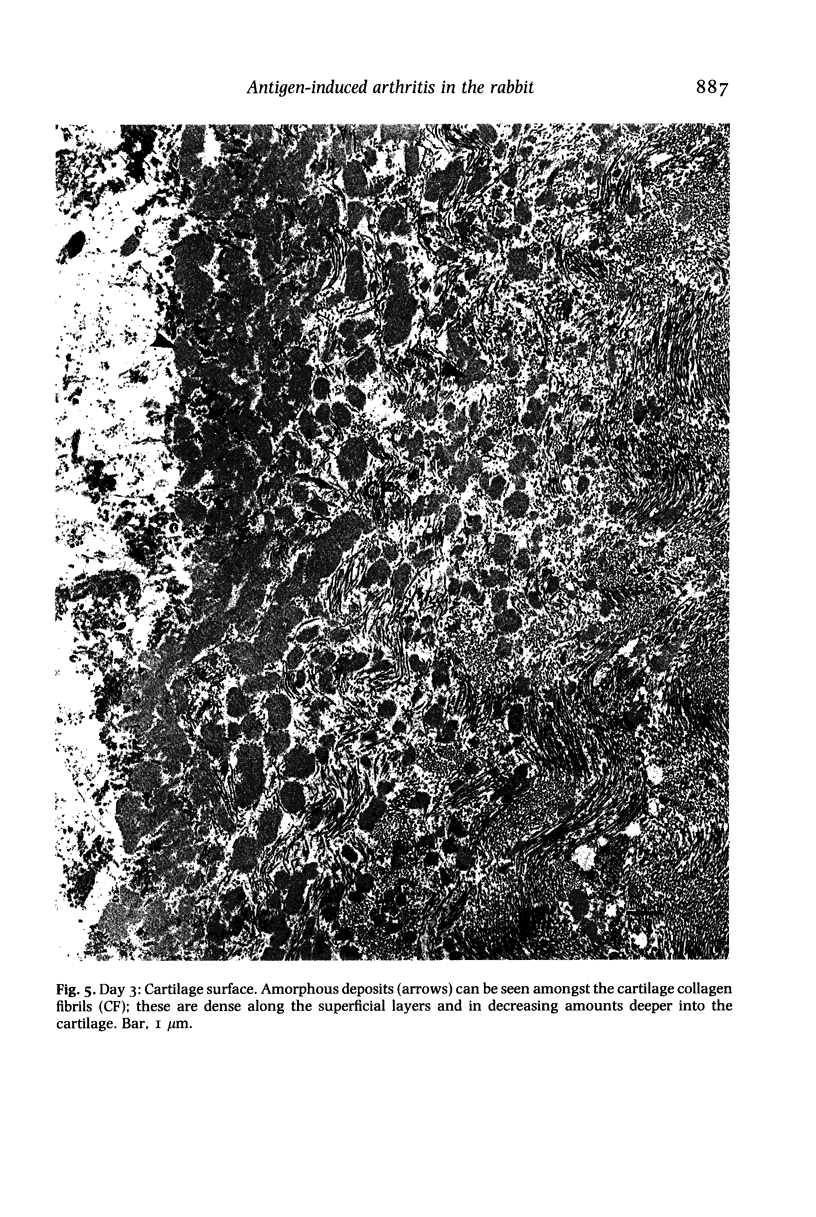
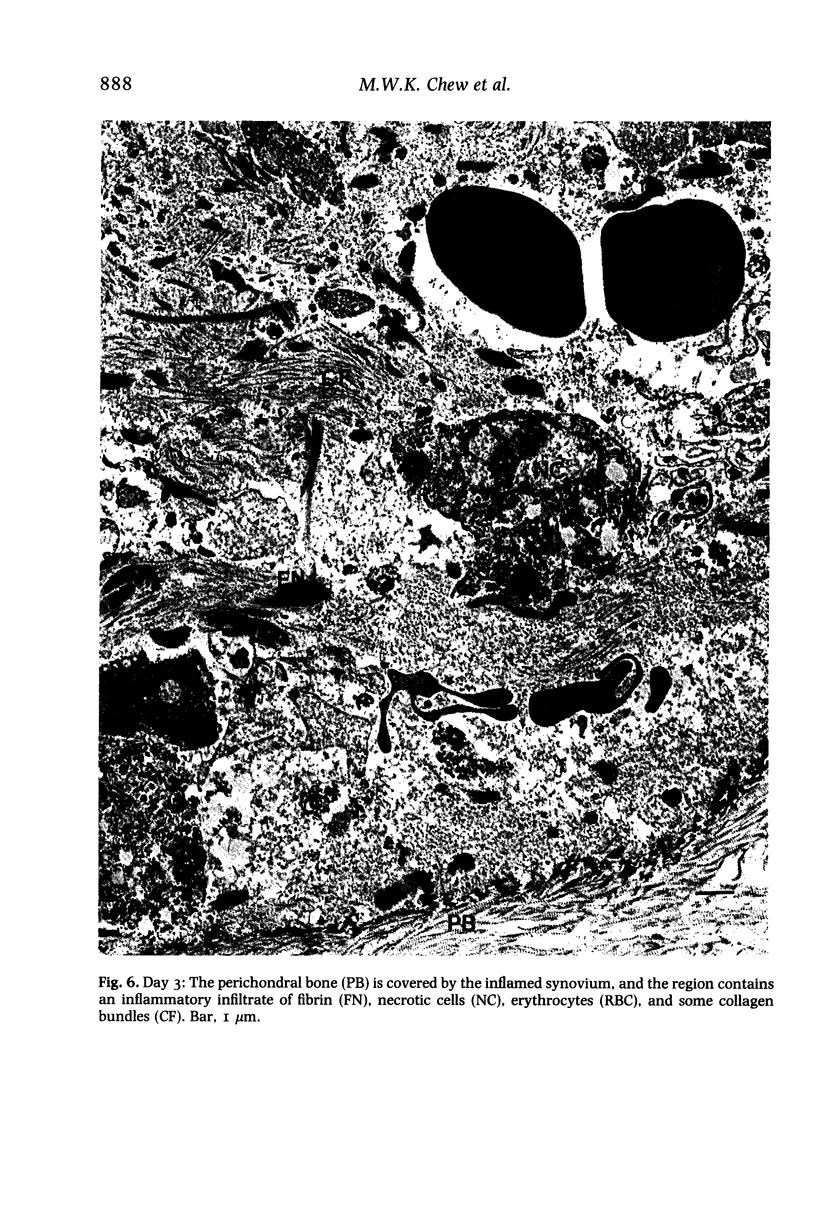
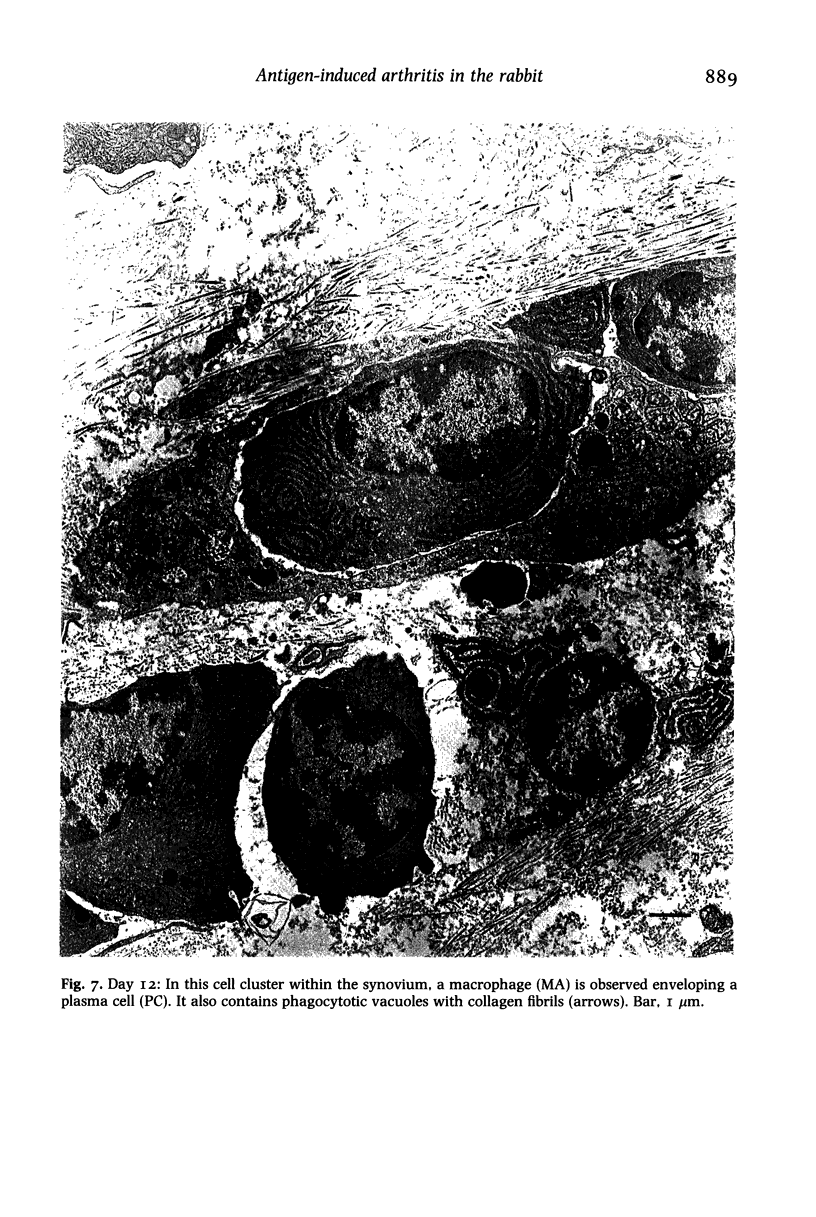
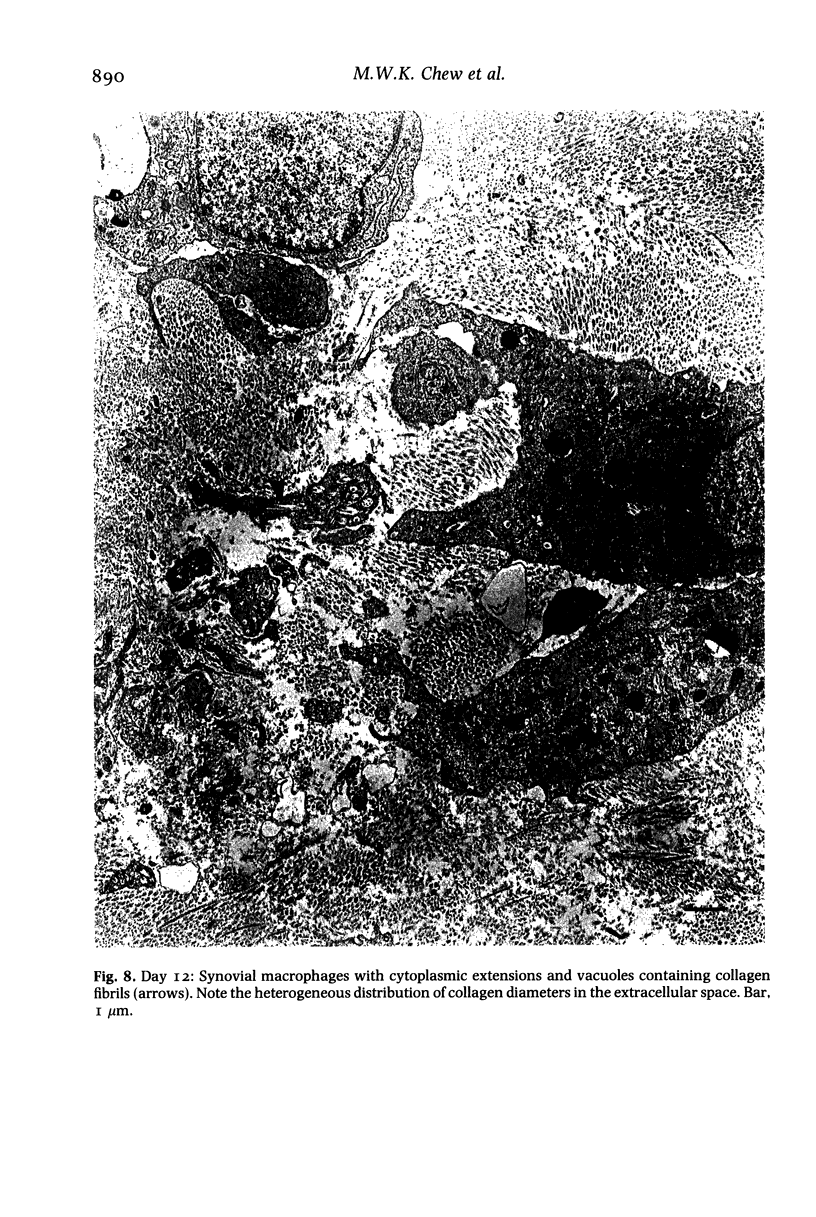
Structural changes at the chondrosynovial junction of the lateral border of the lateral femoral condyle have been studied by electron microscopy in rabbits with antigen-induced arthritis of 6 h-27 days duration. Rapid changes in the collagen fibrils of the extracellular matrix in the synovial lining and articular cartilage were noted. Collagen fibrils with unusually large diameters were observed. Overgrowth of cartilage by inflamed synovium was seen within 3-6 days of induction of arthritis and by day 12 the interface between these two tissues was largely indistinguishable. The synovial pannus at this time was fibrotic and infiltrated with plasma cells, lymphocytes and macrophages. Few polymorphonuclear leucocytes were found in the developing pannus. Macrophages were found with extended processes which enveloped neighbouring cells. Some blood vessels had thickened endothelial cells though lymphocytes were not observed in their vicinity. This study reveals the rapidity with which synovial pannus can develop and suggests that there are a number of mechanisms operating to cause cartilage breakdown in antigen-induced arthritis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrie H. J. Histologic changes in rheumatoid disease of the metacarpal and metatarsal heads as seen in surgical material. J Rheumatol. 1981 Mar-Apr;8(2):246–257. [PubMed] [Google Scholar]
- Bromley M., Woolley D. E. Histopathology of the rheumatoid lesion. Identification of cell types at sites of cartilage erosion. Arthritis Rheum. 1984 Aug;27(8):857–863. doi: 10.1002/art.1780270804. [DOI] [PubMed] [Google Scholar]
- DUMONDE D. C., GLYNN L. E. The production of arthritis in rabbits by an immunological reaction to fibrin. Br J Exp Pathol. 1962 Aug;43:373–383. [PMC free article] [PubMed] [Google Scholar]
- Harding B., Knight S. C. The distribution of dendritic cells in the synovial fluids of patients with arthritis. Clin Exp Immunol. 1986 Mar;63(3):594–600. [PMC free article] [PubMed] [Google Scholar]
- Henderson B., Glynn L. E. Metabolic alterations in the synoviocytes in chronically inflamed knee joints in immune arthritis in the rabbit: comparison with rheumatoid arthritis. Br J Exp Pathol. 1981 Feb;62(1):27–33. [PMC free article] [PubMed] [Google Scholar]
- Henderson B., Higgs G. A. Synthesis of arachidonate oxidation products by synovial joint tissues during the development of chronic erosive arthritis. Arthritis Rheum. 1987 Oct;30(10):1149–1156. doi: 10.1002/art.1780301010. [DOI] [PubMed] [Google Scholar]
- Henderson B. Increase in the activity of lysosomal acid hydrolases in the chondrocytes of arthritic joints of rabbits with experimental allergic arthritis. Histochem J. 1984 Mar;16(3):287–293. doi: 10.1007/BF01003612. [DOI] [PubMed] [Google Scholar]
- Iguchi T., Ziff M. Electron microscopic study of rheumatoid synovial vasculature. Intimate relationship between tall endothelium and lymphoid aggregation. J Clin Invest. 1986 Feb;77(2):355–361. doi: 10.1172/JCI112312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz E. P., Wachtel E. J., Maroudas A. Extrafibrillar proteoglycans osmotically regulate the molecular packing of collagen in cartilage. Biochim Biophys Acta. 1986 Jun 3;882(1):136–139. doi: 10.1016/0304-4165(86)90065-6. [DOI] [PubMed] [Google Scholar]
- Kobayashi I., Ziff M. Electron microscopic studies of the cartilage-pannus junction in rheumatoid arthritis. Arthritis Rheum. 1975 Sep-Oct;18(5):475–483. doi: 10.1002/art.1780180507. [DOI] [PubMed] [Google Scholar]
- Pettipher E. R., Henderson B., Edwards J. C., Higgs G. A. Effect of indomethacin on swelling, lymphocyte influx, and cartilage proteoglycan depletion in experimental arthritis. Ann Rheum Dis. 1989 Aug;48(8):623–627. doi: 10.1136/ard.48.8.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettipher E. R., Henderson B., Hardingham T., Ratcliffe A. Cartilage proteoglycan depletion in acute and chronic antigen-induced arthritis. Arthritis Rheum. 1989 May;32(5):601–607. doi: 10.1002/anr.1780320514. [DOI] [PubMed] [Google Scholar]
- Pettipher E. R., Henderson B. The relationship between cell-mediated immunity and cartilage degradation in antigen-induced arthritis in the rabbit. Br J Exp Pathol. 1988 Feb;69(1):113–122. [PMC free article] [PubMed] [Google Scholar]
- Weibel E. R. Stereological principles for morphometry in electron microscopic cytology. Int Rev Cytol. 1969;26:235–302. doi: 10.1016/s0074-7696(08)61637-x. [DOI] [PubMed] [Google Scholar]