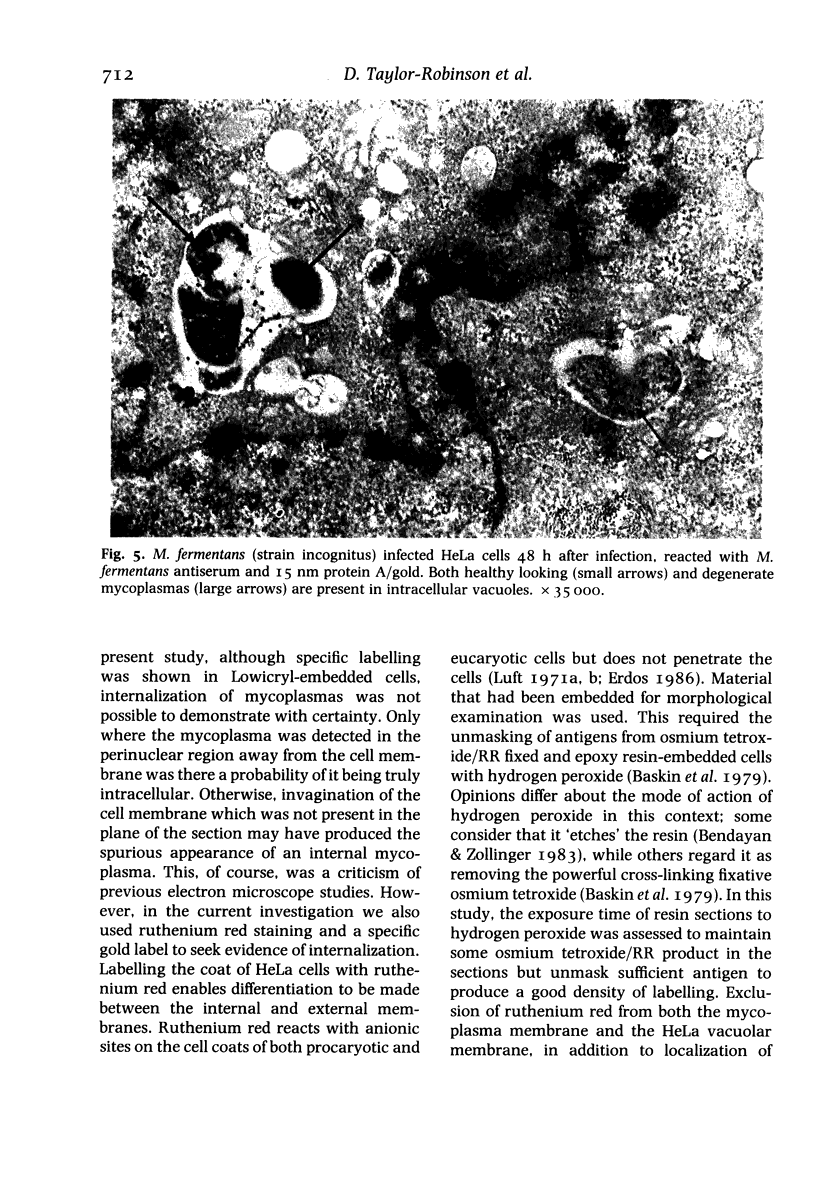
Abstract
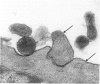
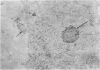
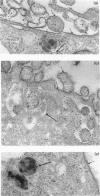
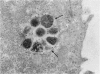
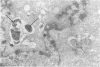
Mycoplasma fermentans (strain 'incognitus') was incubated with HeLa cells for up to 96 h. After 24 h, mycoplasma organisms were demonstrated intracellularly by immunocytochemistry using mule anti-M. fermentans antiserum and gold labelling on ultrathin sections of both Lowicryl K4M and Araldite-embedded HeLa cells, the latter being treated with hydrogen peroxide. The Araldite-embedded cells were fixed with glutaraldehyde and osmium tetroxide in the presence of ruthenium red to stain the mucopolysaccharide surface components of both the procaryotic and eucaryotic cells. Intracellular localization of some M. fermentans organisms was confirmed by exclusion of ruthenium red from their membranes. Various numbers of mycoplasma organisms were seen per cell and occasionally some were within vacuoles, the membranes of which were also unstained by ruthenium red. The PG18 strain of M. fermentans and a strain of M. hominis were also detected intracellularly using similar methodology and homologous mule or rabbit antisera. The occasional presence of both apparently normal and some denser degenerate mycoplasmas in the same cell may indicate gradual degradation by phagolysosomal digestion.
Full text
PDF



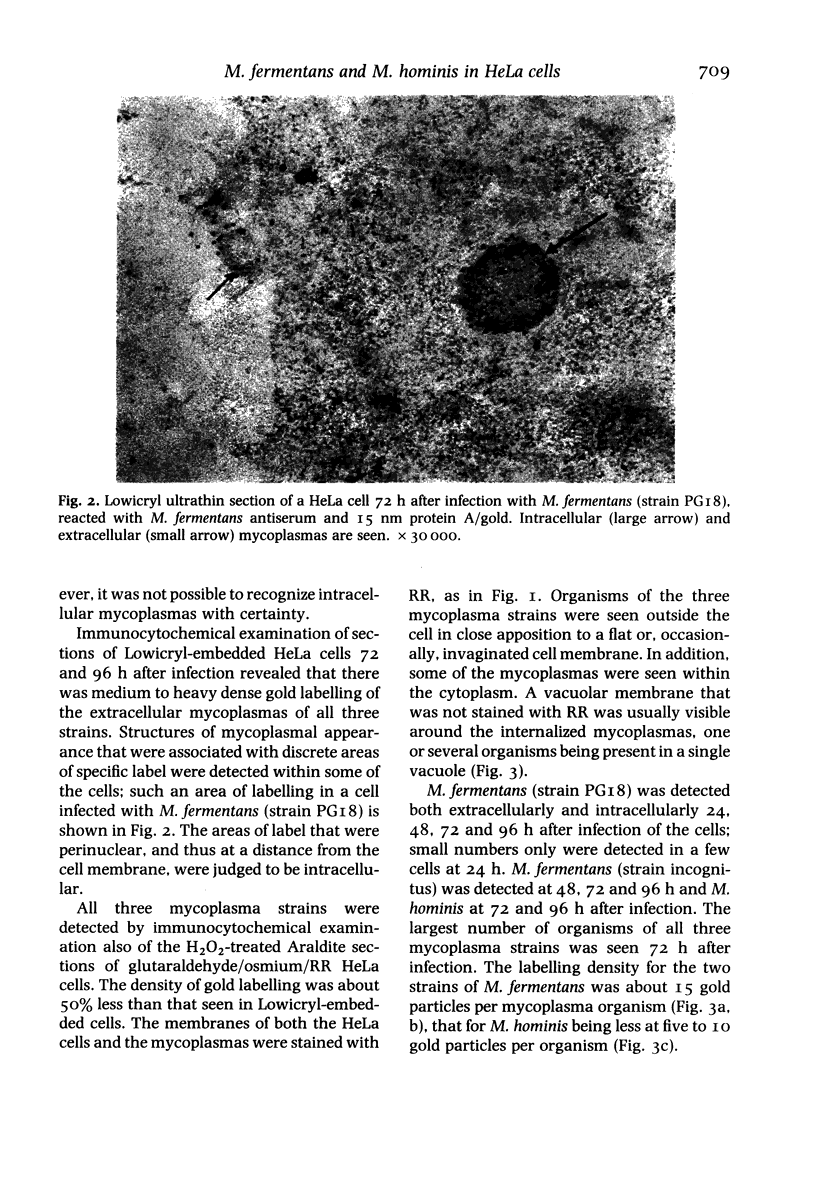
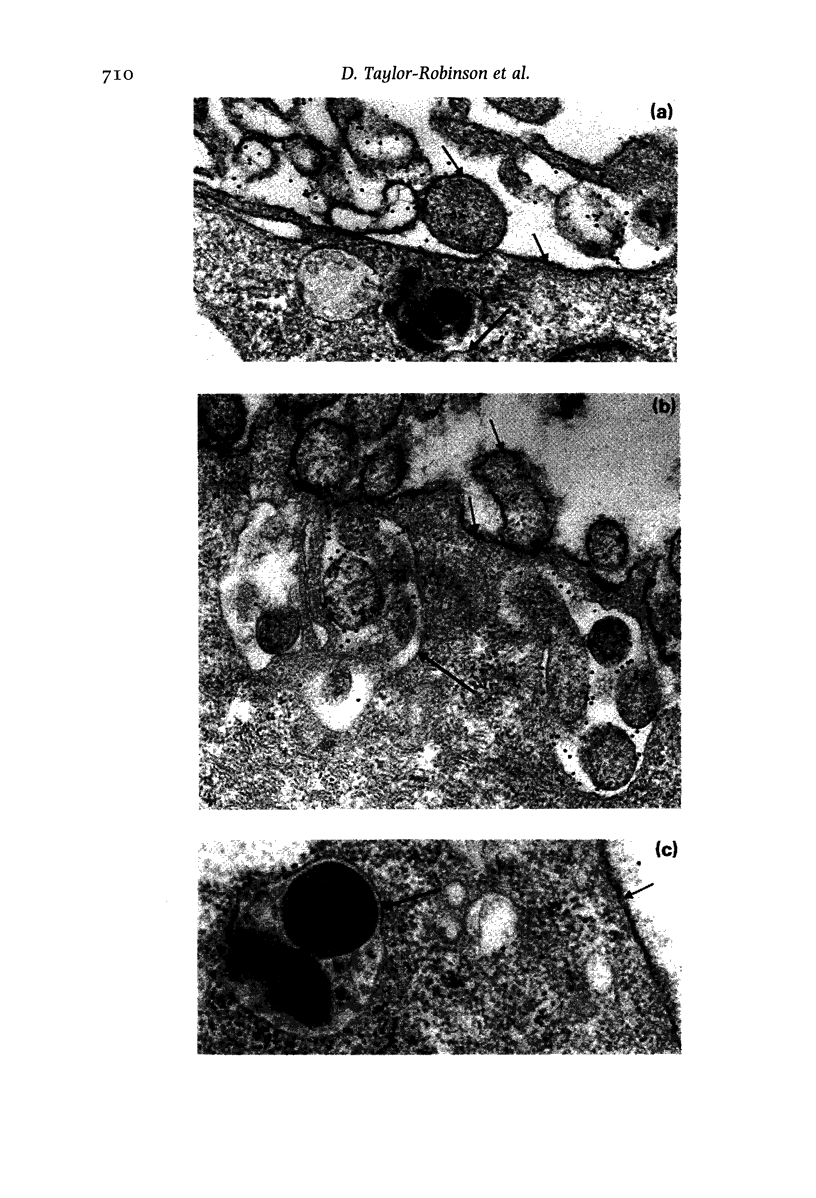
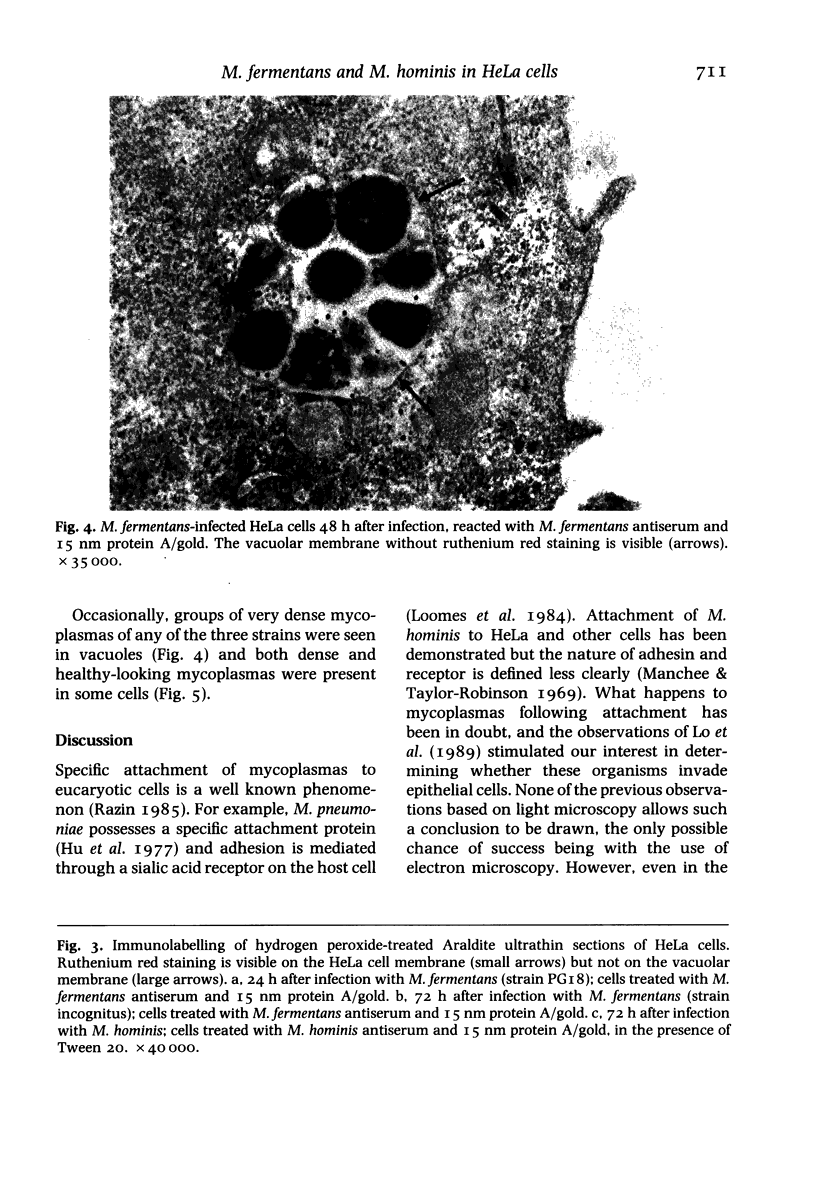


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendayan M., Zollinger M. Ultrastructural localization of antigenic sites on osmium-fixed tissues applying the protein A-gold technique. J Histochem Cytochem. 1983 Jan;31(1):101–109. doi: 10.1177/31.1.6187796. [DOI] [PubMed] [Google Scholar]
- EDWARDS G. A., FOGH J. Fine structure of pleuropneumonia-like organisms in pure culture and in infected tissue culture cells. J Bacteriol. 1960 Feb;79:267–276. doi: 10.1128/jb.79.2.267-276.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogh J., Fogh H. Morphological and quantitative aspects of mycoplasma-human cell relationships. Proc Soc Exp Biol Med. 1967 Jun;125(2):423–430. doi: 10.3181/00379727-125-32110. [DOI] [PubMed] [Google Scholar]
- Frehel C., de Chastellier C., Lang T., Rastogi N. Evidence for inhibition of fusion of lysosomal and prelysosomal compartments with phagosomes in macrophages infected with pathogenic Mycobacterium avium. Infect Immun. 1986 Apr;52(1):252–262. doi: 10.1128/iai.52.1.252-262.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYFLICK L., STINEBRING W. R. Intracellular growth of pleuropneumonialike organisms (PPLO) in tissue culture and in ovo. Ann N Y Acad Sci. 1960 Jan 15;79:433–449. doi: 10.1111/j.1749-6632.1960.tb42709.x. [DOI] [PubMed] [Google Scholar]
- Hale T. L., Bonventre P. F. Shigella infection of Henle intestinal epithelial cells: role of the bacterium. Infect Immun. 1979 Jun;24(3):879–886. doi: 10.1128/iai.24.3.879-886.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Morris R. E., Bonventre P. F. Shigella infection of henle intestinal epithelial cells: role of the host cell. Infect Immun. 1979 Jun;24(3):887–894. doi: 10.1128/iai.24.3.887-894.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P. C., Collier A. M., Baseman J. B. Surface parasitism by Mycoplasma pneumoniae of respiratory epithelium. J Exp Med. 1977 May 1;145(5):1328–1343. doi: 10.1084/jem.145.5.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomes L. M., Uemura K., Childs R. A., Paulson J. C., Rogers G. N., Scudder P. R., Michalski J. C., Hounsell E. F., Taylor-Robinson D., Feizi T. Erythrocyte receptors for Mycoplasma pneumoniae are sialylated oligosaccharides of Ii antigen type. Nature. 1984 Feb 9;307(5951):560–563. doi: 10.1038/307560a0. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. II. Fine structural localization in animal tissues. Anat Rec. 1971 Nov;171(3):369–415. doi: 10.1002/ar.1091710303. [DOI] [PubMed] [Google Scholar]
- Moulder J. W. Comparative biology of intracellular parasitism. Microbiol Rev. 1985 Sep;49(3):298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orihel T. C. Filariasis then. Am J Trop Med Hyg. 1989 Sep;41(3 Suppl):6–8. doi: 10.4269/ajtmh.1989.41.6. [DOI] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Carlemalm E., Villiger W., Garavito M. Enhancement of structural preservation and immunocytochemical staining in low temperature embedded pancreatic tissue. J Histochem Cytochem. 1981 May;29(5):663–671. doi: 10.1177/29.5.6166664. [DOI] [PubMed] [Google Scholar]
- Shedden W. I., Cole B. C. Rapid method for demonstrating intracellular pleuropneumonia-like organisms in a strain of hamster kidney cells (BHK 21 C13). Nature. 1966 May 21;210(5038):868–868. doi: 10.1038/210868a0. [DOI] [PubMed] [Google Scholar]
- Taylor-Robinson D., Furr P. M. Recovery and identification of human genital tract mycoplasmas. Isr J Med Sci. 1981 Jul;17(7):648–653. [PubMed] [Google Scholar]
- Zucker-Franklin D., Davidson M., Thomas L. The interaction of mycoplasmas with mammalian cells. I. HeLa cells, neutrophils, and eosinophils. J Exp Med. 1966 Sep 1;124(3):521–532. doi: 10.1084/jem.124.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]