Abstract
The middle urethra and external urethral sphincter are the focus in management of stress urinary incontinence, and recent cellular-therapy research suggests a new paradigm in treatment. Cell-based therapies are most often described as using autologous multipotent stem cells procured from bone marrow in procedures that may be painful, require anesthesia, and yield low numbers of mesenchymal stem cells upon processing. In contrast, muscleand adipose-derived stem cells can be obtained easily in large quantities under local anesthesia. Instead of lifting the urethra with a sling or bulking up the urethral sphincter with collagen, we now have the potential to restore function with the use of autologous stem cells.
Key words: Urinary incontinence, Urethral sphincter, Muscle-derived stem cells, Adipose-derived stem cells
More than 200 million people worldwide live with incontinence.1 Incontinence occurs frequently from middle age onward and is associated with a reduced quality of life.2 The yearly direct cost of urinary incontinence in the United States alone is $16.3 billion, of which three-quarters is for the management of women who have the condition.3 Stress urinary incontinence (SUI) is the most common type of urinary incontinence.4 Most studies of risk factors for SUI have included cross-sectional studies, with the best-studied factors being parity, age, and obesity.5 Childbirth injury to muscles, connective tissue, and nerves seems to be the most important risk factor for lifetime incontinence in specific women.6,7
Pharmacological treatment of SUI, such as α-adrenoceptor agonists, has been disappointing.8 Recently, duloxetine, a selective serotonin and norepinephrine reuptake inhibitor, was not approved by the US Food and Drug Administration (FDA) for the indication of SUI.9 Injection of bulking agents is regarded as a less invasive method to treat SUI compared with sling or Burch procedures. The use of injectable bulking agents, such as Teflon® (DuPont, Wilmington, DE), bovine collagen, silicone particles, and carbon beads, has yielded some short-term success, but these agents can cause chronic inflammation, foreign-body giant-cell response, periurethral abscess, erosion of the urinary bladder or the urethra, obstruction of the lower urinary tract with resultant urinary retention, severe voiding dysfunction, migration to inner organs, and pulmonary embolism.10 Autologous ear chondrocytes also have been used as bulking agents for the treatment of SUI.11
Stem-cell therapy for the potential cure of the deficient sphincter in SUI has been at the forefront of incontinence research.12 Researchers have attempted to use cell-based therapies to regenerate the deficient muscle and connective tissue that results in SUI. The aim of stem-cell therapy is to replace, repair, or enhance the biological function of damaged tissue or organs. The 2 general types of stem cells that are potentially useful for the treatment are embryonic stem cells (ESCs) and adult stem cells. Although theoretically appealing, the practical use of ESCs is limited due to potential regulatory problems and ethical considerations. In contrast, no significant ethical issues are associated with the use of adult stem cells.
This is our vision for the near future: A man or woman with SUI comes into the urologist’s or urogynecologist’s office, where the lateral thigh is infiltrated with lidocaine and a skin puncture incision is made using a muscle needle biopsy device similar to what is used for prostate biopsy. Then a core of muscle is harvested and placed in a sterile container. The biopsy container is stored in ice and express-mailed to a central FDAapproved stem-cell facility for processing. At the stem-cell facility, the biopsy specimen is dissociated and muscle-derived stem cells (MDSCs) are isolated. An adequate number of MDSCs-up to 100 million cells-can be prepared within approximately 3 weeks, and the cells can be stored indefinitely. When the patient is ready and makes an appointment with the doctor’s office, the MDSCs are checked back, frozen, and reconstituted with saline before injection. Using a cystoscopy or spinal needle, the MDSCs are injected into the patient’s urethral sphincter under local anesthesia in the doctor’s office (Figure 1).
Figure 1.
Diagram showing autologous muscle-derived stem cells injection therapy for stress urinary incontinence (SUI). Autologous stem cells are obtained from a biopsy of tissue, the cells are dissociated and expanded in culture, and the expanded cells are implanted into the same host.
Neurophysiology
The urethral muscles are controlled by 3 sets of peripheral nerves: (1) sacral parasympathetic nerves (pelvic nerves), (2) thoracolumbar sympathetic nerves (hypogastric nerves and sympathetic chain), and (3) sacral somatic nerves (pudendal nerves). Sympathetic preganglionic pathways emerge from the thoracolumber spinal cord and pass to the sympathetic chain ganglia and then the inferior splanchnic nerves to the inferior mesenteric ganglia. Preganglionic and postganglionic sympathetic axons then travel in the hypogastric nerve to the pelvic plexus and the urogenital organs. Parasympathetic preganglionic axons that originate in the sacral spinal cord pass in the pelvic nerve to ganglion cells in the pelvic plexus and to distal ganglia in the organs. Sacral somatic pathways are contained in the pudendal nerve, which provides an innervation to the external urethral sphincter (EUS) and the penis. The pudendal and pelvic nerves also receive postganglionic axons from the caudal sympathetic chain ganglia. These 3 sets of nerves contain afferent axons from the lumbosacral dorsal root ganglia.13
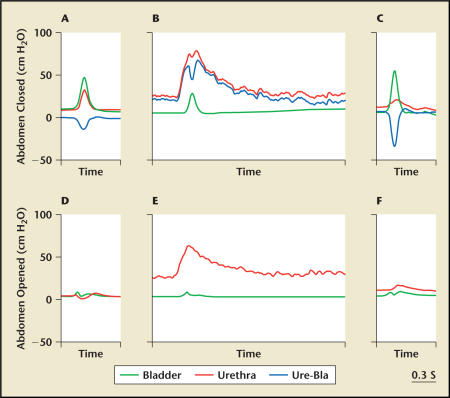
The EUS can be activated voluntarily or by reflex mechanism elicited by bladder distension. Nerve tracts from the central nervous system terminate at the Onuf’s nucleus in the sacral spinal cord and synapse with the pudendal nerves. Serotonin and norepinephrine are 2 key neurotransmitters that stimulate the Onuf’s nucleus of the pudendal nerves to control the contraction of the EUS.14,15 Nervemediated urethral closure mechanisms that maintain urinary continence during elevation of abdominal pressure may be divided into the 3 groups: central nervous control passing to Onuf’s nucleus,16 bladder-to-urethral reflex,17,18 and resting tone of the urethra.18 Kamo and colleagues16,18 showed in female rats that (1) the middle urethral increase under sneezing was caused not only by passive transmission of increased abdominal pressure but also by active reflex contraction of urethral sphincter and pelvic floor muscles, whereas the proximal and distal urethral increase under sneezing was dependent on intravesicular or intra-abdominal pressure (Figure 2); (2) passive elevation of intravesicular pressure elicited pelvic afferent nerve-mediated contractile reflex, bladder-to-urethral reflex, in the middle-to-proximal urethra mediated by the activation of hypogastric and pudendal nerves (Figure 3); and (3) the urethral baseline pressure was highest in the middle portion of the urethra located at 10 mm to 12.5 mm from the urethral orifice (Figure 3). These experimental data suggest that the middle urethra and EUS are critical for maintaining continence and a primary focus for the management of SUI.
Figure 2.
Expanded recordings by microtip transducers showing responses in the bladder and urethra during sneezing under abdomen-closed (A–C) or abdomen-open (D–F) conditions in rats. Urethral responses were measured at the proximal (A, D), middle (B, E), and distal (C, F) parts of the urethra. Ure-Bla, bladder and urethral response difference. Adapted from Kamo et al.16
Figure 3.
Changes in urethral pressure responses at different sites in the urethra (2.5-mm steps from the urethral orifice) measured by a microtip transducer catheter during increments in intravesicular pressure to 20, 40, or 60 cm H2O in female rats. Note that the intravesicular pressure elevation-induced urethral response was the biggest in the urethra at 12.5 mm to 15 mm, whereas the baseline was the highest in the urethra at 10 mm to 12.5 mm from the orifice. Adapted from Kamo et al.18
Stem-Cell Source for Injection Therapy of SUI
Cell-based therapies and tissue engineering are most often described with the use of autologous multipotent stem cells, of which one such commonly described source consists of bone marrow stromal cells. The bone marrow compartment contains several cell populations, including mesenchymal stem cells (MSCs) that are capable of differentiating into adipogenic,19 osteogenic,19 chondrogenic,20 and myogenic cells.21,22 Autologous bone marrow procurement, however, has significant potential limitations because the procedure may be painful, frequently requiring general or spinal anesthesia, and may yield low numbers of MSCs upon processing.19 As an alternative source of autologous adult stem cells, MDSCs and adipose-derived stem cells (ADSCs) are advantageous because they can be obtained easily in large quantities under local anesthesia.
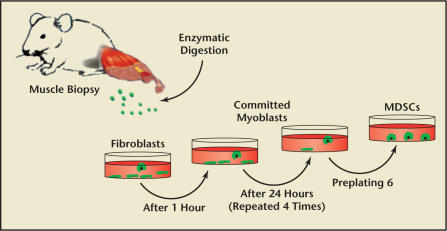
Myoblast transfer therapy, however, has numerous limitations, including immunological problems and low spread and poor survival of the injected myoblasts. Selection of MDSCs population using the preplating technique can be regarded as an approach to improve cell survival after transplantation (Figure 4).23 Moreover, in comparison with satellite cells in muscles, MDSCs display an improved transplantation capacity-that is, the ability to undergo longterm proliferation, self-renewal, and multipotent differentiation, including differentiation toward endothelial and neuronal lineages.24,25
Figure 4.
Preplating technique. The isolated cells are preplated in collagen-coated flasks. After 1 hour, the supernatant is withdrawn from the flask and replated in a fresh collagen-coated flask. The cells that adhered rapidly within 1-hour incubation are mostly fibroblasts. The serial replating of the supernatant is repeated when 30% to 40% of the cells have adhered to each flask. After 5 to 6 serial platings, the culture is enriched with muscle-derived stem cells (MDSCs).
MDSCs injection therapy has several advantages over the current treatment of SUI. First, cells that are derived from incontinence patients and injected back into them (autologous cell transplantation) will not cause an immunogenic or allergic reaction and therefore survive longer than injected collagen.26,27 Second, cells transplanted into the urethra can serve as a natural bulking agent like autologous ear chondrocytes. Finally, these cells form myotubes and myofibers that become innervated into the host muscle. Therefore, they not only serve as a blocking agent but also are physiologically capable of improving urethral function.28–30
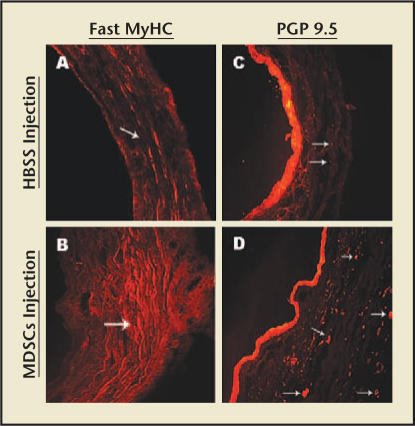
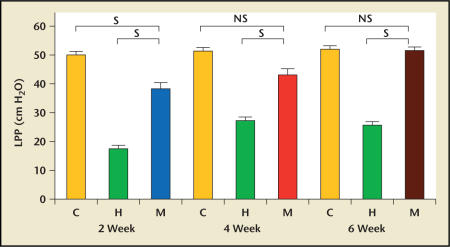
The feasibility of this concept was demonstrated in a rat model of SUI.12,31 Chermansky and colleagues32 showed that MDSCs had integrated within the striated muscle layer of the cauterized middle urethra 4 weeks after MDSCs injection. The striated muscle layer of the MDSCs-injected urethra was contiguous and had more nerves than the cauterized urethra injected with only Hank’s balanced salt solution (HBSS) (Figure 5). These results support the finding that MDSCs have the ability of mutipotent differentiation in the host tissue. In addition, the increase in leak point pressure (LPP) seen in the groups injected with MDSCs was significant when compared with the cauterized rats injected with HBSS. The difference in LPP seen in the groups 4 and 6 weeks after the MDSCs injection was not significant when compared with control normal rats (Figure 6).
Figure 5.
Differences in striated muscle layer and innervation of cauterized midurethra in rats. (A) Fast myosin heavy chain (MyHC) stain of cauterized midurethra 4 weeks after Hank’s balanced salt solution (HBSS) injection. Arrow points to the disrupted striated muscle layer. (B) Fast MyHC stain of cauterized mid-urethra 4 weeks after the musclederived stem cells (MDSCs) injection. Arrow points to the intact striated muscle layer. (C) PGP 9.5 stain of cauterized mid-urethra 4 weeks after the HBSS injection. Arrows point to the few nerve fibers present. (D) PGP 9.5 stain of cauterized mid-urethra 4 weeks after the MDSCs injection. Arrows point to many nerve fibers present. Magnification, ×200. Adapted from Chermansky et al.32
Figure 6.
Compared with cauterized rats injected with Hank’s balanced salt solution (HBSS) and matched respective to time, increased leak point pressures (LPPs) seen in muscle-derived stem cell (MDSCs) injected groups were significantly higher. Compared with control rats (C) and matched respective to time, LPPs seen in the MDSCsinjected groups 4 and 6 weeks after the MDSCs injection were not statistically different. C, control group; H, HBSSinjected group; M, MDSCs-injected group; S, statistically significant; NS, not statistically significant. Adapted from Chermansky et al.32
Zuk and associates,33 however, reported that ADSCs can differentiate in vitro into adipogenic, myogenic, and osteogenic cells in the presence of lineage-specific induction factors. In addition, ADSCs exhibit the functional ability to contract and relax in direct response to pharmacologic agents.34 ADSCs may also represent an alternative stem-cell source for the treatment of SUI.35 The feasibility of the use of ADSCs was indicated by improvements in LPP and urethral function in a rat model of SUI when animals were injected with ADSCs in conjunction with biodegradable microbeads as a carrier.36 As a cost-effective source for genitourinary reconstruction, MDSCs and ADSCs offer a promising technology.
Current Results of Clinical Studies
In an abstract, Strasser and colleagues37 reported results from their initial 130 patients with SUI in a clinical trial. They used a transurethral probe to inject fibroblasts mixed with collagen into the urethral submucosa to treat atrophies of the mucosa and myoblasts into the rhabdosphincter to reconstruct the muscle under an ultrasound guidance technique.38 The myoblasts and fibroblasts were obtained from upper-arm biopsy. Eighty-five percent of the patients were cured of incontinence, and thickness of the urethra and rhabdosphincter were increased significantly at short-term follow-up. The fractional benefit of the myoblasts versus fibroblasts versus the 2.5 mL of collagen used in the mixed cellular-plus-collagen injection approach is unclear.
In an abstract presented at the 2006 American Urological Association annual meeting, Carr and colleagues39 reported on pure cellular clinical therapy with MDSCs obtained from biopsies of the lateral thigh. They were the first to treat North American patients. Six patients had received treatment using either a transurethral or a periurethral injection into the middle urethra and EUS. The 2 transurethral injections using a 10-mm needle and the 2 periurethral injections resulted in measurable improvement, but the treatment consisting of 2 initial injections with an 8-mm needle was not effective. The technique of the MDSCs injection should be optimized.
Conclusion
Most authorities regard the suburethral sling operation as the gold standard in SUI treatment. The sling procedure functions as a hammock under the middle urethra to reinforce the weakness of levator ani muscles and supportive ligaments or fascia, whereas stem cell injection therapy into the middle urethra has the potential to restore the contractile response of the striated muscle and rhabdosphincter. A multicenter clinical trial with autologous MDSCs is under way in Canada.
Main Points.
Pharmacological treatment of stress urinary incontinence (SUI) has been disappointing.
Injectable bulking agents have yielded some short-term success but can cause chronic inflammation and other adverse events.
Stem-cell therapy offers a potential cure of the deficient sphincter by regenerating damaged muscle and connective tissue.
Experimental data suggest that the middle urethra and external urethral sphincter are critical for maintaining continence and a primary focus for the management of SUI.
As an alternative source of autologous adult stem cells, muscle-derived stem cells (MDSCs) are advantageous because, in comparison with bone-marrow cells, they can be obtained easily in large quantities under local anesthesia.
MDSCs injection therapy has several advantages over current treatment of SUI. Autologous cell transplantation will not cause an immunogenic or allergic reaction. Cells transplanted into the urethra can serve as a natural bulking agent and become innervated into the host muscle, potentially improving urethral function.
The current gold standard for SUI treatment, suburethral sling operation, reinforces muscles and ligaments of the middle urethra, whereas stem-cell injection therapy has the potential to restore the contractile response of the striated muscle and rhabdosphincter.
References
- 1.Norton P, Brubaker L. Urinary incontinence in women. Lancet. 2006;367:57–67. doi: 10.1016/S0140-6736(06)67925-7. [DOI] [PubMed] [Google Scholar]
- 2.Corcos J, Beaulieu S, Donovan J. Quality of life assessment in men and women with urinary incontinence. J Urol. 2002;168:896–905. doi: 10.1016/S0022-5347(05)64540-5. [DOI] [PubMed] [Google Scholar]
- 3.Wilson L, Brown JS, Shin GP. Annual direct cost of urinary incontinence. Obstet Gynecol. 2001;98:398–406. doi: 10.1016/s0029-7844(01)01464-8. [DOI] [PubMed] [Google Scholar]
- 4.Hampel C, Wienhold D, Benken N. Prevalence and natural history of female incontinence. Eur Urol. 1997;32(2 suppl):3–12. [PubMed] [Google Scholar]
- 5.Bump RC, Norton PA. Epidemiology and natural history of pelvic floor dysfunction. Obstet Gynecol Clin North Am. 1998;25:723–746. doi: 10.1016/s0889-8545(05)70039-5. [DOI] [PubMed] [Google Scholar]
- 6.Meyer S, Schreyer A, De Grandi P. The effects of birth on urinary continence mechanisms and other pelvic-floor characteristics. Obstet Gynecol. 1998;92:613–618. doi: 10.1016/s0029-7844(98)00248-8. [DOI] [PubMed] [Google Scholar]
- 7.Sampselle CM, Miller JM, Mims BL. Effect of pelvic muscle exercise on transient incontinence during pregnancy and after birth. Obstet Gynecol. 1998;91:406–412. doi: 10.1016/s0029-7844(97)00672-8. [DOI] [PubMed] [Google Scholar]
- 8.Radley SC, Chapple CR, Bryan NP. Effect of methoxamine on maximum urethral pressure in women with genuine stress incontinence: a placebo-controlled, double-blind crossover study. Neurourol Urodyn. 2001;20:43–52. doi: 10.1002/1520-6777(2001)20:1<43::aid-nau6>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 9.Chancellor MB. Duloxetine for treatment of stress urinary incontinence. Evid Based OBGYN. 2004;6:196–199. [Google Scholar]
- 10.Kiilholma PJ, Chancellor MB, Makinen J. Complications of Teflon injection for stress urinary incontinence. Neurourol Urodyn. 1993;12:131–137. doi: 10.1002/nau.1930120206. [DOI] [PubMed] [Google Scholar]
- 11.Bent AE, Tutrone RT, McLennan MT. Treatment of intrinsic sphincter deficiency using autologous ear chondrocytes as a bulking agent. Neurourol Urodyn. 2001;20:157–165. doi: 10.1002/1520-6777(2001)20:2<157::aid-nau18>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 12.Lee JY, Cannon TW, Pruchnic R. The effects of periurethral muscle-derived stem cell injection on leak point pressure in a rat model of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2003;14:31–37. doi: 10.1007/s00192-002-1004-5. [DOI] [PubMed] [Google Scholar]
- 13.de Groat WC. Integrative control of the lower urinary tract: preclinical perspective. Br J Pharmacol. 2006;147(2 suppl):S25–S40. doi: 10.1038/sj.bjp.0706604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thor KB, Katofiasc MA. Effects of duloxetine, a combined serotonin and norepinephrine reuptake inhibitor, on central neural control of lower urinary tract function in the chloralose-anesthetized female cat. J Pharmacol Exp Ther. 1995;274:1014–1024. [PubMed] [Google Scholar]
- 15.Chancellor MB, Perkin H, Yoshimura N. Recent advances in the neurophysiology of stress urinary incontinence. Scand J Urol Nephrol. 2005;39:21–24. doi: 10.1080/00365590410002474. [DOI] [PubMed] [Google Scholar]
- 16.Kamo I, Torimoto K, Chancellor MB. Urethral closure mechanisms under sneeze-induced stress condition in rats: a new animal model for evaluation of stress urinary incontinence. Am J Physiol Regul Integr Comp Physiol. 2003;285:R356–R365. doi: 10.1152/ajpregu.00010.2003. [DOI] [PubMed] [Google Scholar]
- 17.de Groat WC. Anatomy of the central neural pathways controlling the lower urinary tract. Eur Urol. 1998;34(1 suppl):2–5. doi: 10.1159/000052265. [DOI] [PubMed] [Google Scholar]
- 18.Kamo I, Cannon TW, Conway DA. The role of bladder-to-urethral reflexes in urinary continence mechanisms in rats. Am J Physiol Renal Physiol. 2004;287:F434–F441. doi: 10.1152/ajprenal.00038.2004. [DOI] [PubMed] [Google Scholar]
- 19.Pittenger MF, Mackay AM, Beck SC. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 20.Ferrari G, Cusella-De Angelis G, Coletta M. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 21.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 22.Dezawa M, Ishikawa H, Itokazu Y. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science. 2005;309:314–317. doi: 10.1126/science.1110364. [DOI] [PubMed] [Google Scholar]
- 23.Qu Z, Balkir L, van Deutekom JC. Development of approaches to improve cell survival in myoblast transfer therapy. J Cell Biol. 1998;142:1257–1267. doi: 10.1083/jcb.142.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qu Z, Deasy B, Jankowski R. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157:851–864. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JY, Qu-Petersen Z, Cao B. Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J Cell Biol. 2000;150:1085–1100. doi: 10.1083/jcb.150.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JY, Paik SY, Yuk SH. Long term effects of muscle-derived stem cells on leak point pressure and closing pressure in rats with transected pudendal nerves. Mol Cells. 2004;18:309–313. [PubMed] [Google Scholar]
- 27.Yokoyama T, Yoshimura N, Dhir R. Persistence and survival of autologous muscle derived cells versus bovine collagen as potential treatment of stress urinary incontinence. J Urol. 2001;165:271–276. doi: 10.1097/00005392-200101000-00077. [DOI] [PubMed] [Google Scholar]
- 28.Chancellor MB, Yokoyama T, Tirney S. Preliminary results of myoblast injection into the urethra and bladder wall: a possible method for the treatment of stress urinary incontinence and impaired detrusor contractility. Neurourol Urodyn. 2000;19:279–287. doi: 10.1002/(sici)1520-6777(2000)19:3<279::aid-nau9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 29.Yokoyama T, Pruchnic R, Lee JY. Autologous primary muscle-derived cells transfer into the lower urinary tract. Tissue Eng. 2001;7:395–404. doi: 10.1089/10763270152436454. [DOI] [PubMed] [Google Scholar]
- 30.Huard J, Yokoyama T, Pruchnic R. Musclederived cell-mediated ex vivo gene therapy for urological dysfunction. Gene Ther. 2002;9:1617–1626. doi: 10.1038/sj.gt.3301816. [DOI] [PubMed] [Google Scholar]
- 31.Cannon TW, Lee JY, Somogyi G. Improved sphincter contractility after allogenic muscle-derived progenitor cell injection into the denervated rat urethra. Urology. 2003;62:958–963. doi: 10.1016/s0090-4295(03)00679-4. [DOI] [PubMed] [Google Scholar]
- 32.Chermansky CJ, Tarin T, Kwon DD. Intraurethral muscle-derived cell injections vd-increase leak point pressure in a rat model of intrinsic sphincter deficiency. Urology. 2004;63:780–785. doi: 10.1016/j.urology.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 33.Zuk PA, Zhu M, Mizuno H. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez LV, Alfonso Z, Zhang R. Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proc Natl Acad Sci USA. 2006;103:12167–12172. doi: 10.1073/pnas.0604850103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jack GS, Almeida FG, Zhang R. Processed lipoaspirate cells for tissue engineering of the lower urinary tract: implications for the treatment of stress urinary incontinence and bladder reconstruction. J Urol. 2005;174:2041–2045. doi: 10.1097/01.ju.0000176489.96993.84. [DOI] [PubMed] [Google Scholar]
- 36.Zeng X, Jack GS, Zhang R. Treatment of SUI using adipose derived stem cells: restoration of urethral function. J Urol. 2006;175 [Google Scholar]
- 37.Strasser H, Marksteiner R, Margreiter E. Transurethral ultrasound guided stem cell therapy of urinary incontinence. J Urol. 2006;175:291. [Google Scholar]
- 38.Strasser H, Pinggera GM, Gozzi C. Threedimensional transrectal ultrasound of the male urethral rhabdosphincter. World J Urol. 2004;22:335–338. doi: 10.1007/s00345-004-0416-x. [DOI] [PubMed] [Google Scholar]
- 39.Carr LK, Steele D, Steele S. Single institution clinical trial of muscle-derived cell injection to treat stress urinary incontinence. J Urol. 2006;175:414. [Google Scholar]