Abstract
Glutathione (GSH), a cysteine-containing tripeptide, is essential for the viability and function of virtually all cells. In vitro studies showing that low GSH levels both promote HIV expression and impair T cell function suggested a link between GSH depletion and HIV disease progression. Clinical studies presented here directly demonstrate that low GSH levels predict poor survival in otherwise indistinguishable HIV-infected subjects. Specifically, we show that GSH deficiency in CD4 T cells from such subjects is associated with markedly decreased survival 2–3 years after baseline data collection (Kaplan–Meier and logistic regression analyses, P < 0.0001 for both analyses). This finding, supported by evidence demonstrating that oral administration of the GSH prodrug N-acetylcysteine replenishes GSH in these subjects and suggesting that N-acetylcysteine administration can improve their survival, establishes GSH deficiency as a key determinant of survival in HIV disease. Further, it argues strongly that the unnecessary or excessive use of acetaminophen, alcohol, or other drugs known to deplete GSH should be avoided by HIV-infected individuals.
Keywords: AIDS, N-acetylcysteine, redox
Glutathione (GSH), like nitric oxide (NO), is a small, ubiquitous molecule that plays key regulatory roles in metabolic and cell-cycle-related functions (1–5). This cysteine-containing tripeptide (γ-glutamylcysteinylglycine), which is found in millimolar concentrations in all animal cells, also provides the principal intracellular defense against oxidative stress (6) and participates in detoxification of many molecules (7). GSH depletion, caused for example by acetaminophen overdose, results in hepatic and renal failure and ultimately in death (7).
HIV-infected people tend to have subnormal GSH levels in plasma (8, 9), lung epithelial lining fluid (10), peripheral blood mononuclear cells (PBMC) (11) and, as determined by measuring GSH as intracellular glutathione-S-bimane fluorescence (GSB) with the fluorescence-activated cell sorter (FACS), in individual CD4 T and other blood cells (12–14). In vitro studies show that lowering intracellular GSH levels decreases cell survival (15), alters T cell functions (16), and increases HIV replication (17, 18), NF-κB activation (17, 18), and sensitivity to tumor necrosis factor-induced cell death (19).
Clinical studies presented here directly link GSH deficiency to impaired survival in HIV disease. We show that (i) the probability that HIV-infected subjects will die within 2–3 years is dramatically higher at low baseline CD4 T cell GSH levels; (ii) oral administration of N-acetylcysteine (NAC), a cysteine prodrug used to replenish GSH after acetaminophen overdose (7), increases GSH levels in HIV-infected subjects; and (iii) this GSH replenishment may be associated with prolongation of survival. Because excessive alcohol consumption (20), UV exposure (21–23), and prolonged or intensive use of medications that deplete GSH—e.g., acetaminophen overdose (7)—can contribute to the GSH depletion in HIV disease, we discuss our findings in terms of changes in medical practice that could slow the development of GSH deficiency and improve survival in HIV disease.§
METHODS
Study Population.
Data presented here are derived from FACS and clinical laboratory assays of blood samples from a cohort of HIV-infected subjects who were screened for enrollment into a clinical trial designed to determine whether orally administered NAC replenishes GSH in subjects with low GSH levels (J.G.D., S.C.D., M.T.A., and L.A.H., unpublished data). None of the screened subjects had CD4 T cell counts above 500/μl blood; none had active opportunistic infections or malignancies; none consumed excessive amounts of products likely to alter GSH levels (e.g., alcohol, vitamin C, vitamin E); none were taking NAC or acetaminophen; and all were readily mobile. Trial enrollment required the following: GSH deficiency indicated by having FACS-measured GSB levels in PBMC CD8 T cells that rank at least one standard deviation below the median for uninfected subjects; no evidence of significant hepatic, renal, or hematologic disease; Karnofsky score >60; and stable (or no) antiretroviral therapy for the previous 4 months (protease inhibitors had not been introduced when these data were collected).
Baseline data represent the average of one to three closely spaced screening measurements. Survival status was ascertained 2–3 years after screening (direct contact for subjects who were still alive; official death records or reliable reports for those who died). Survival data were obtained for 204/246 screened subjects (107/135 with CD4 ≥ 200/μl; 97/111 with CD4 < 200/μl). Six subjects who developed opportunistic infections within 2 weeks of screening were excluded.
Uninfected control subjects were similar in age to the HIV-infected group. About half were laboratory personnel; the remainder lived in the vicinity of the HIV-infected subjects. The percentage of female subjects was higher (≈30%) in the control group.
NAC Administration.
All subjects who took NAC in studies reported here were randomized to receive either NAC or placebo during the double-blind phase of a clinical trial testing oral NAC for GSH replenishment in HIV-infected subjects. All subjects completing this initial 8-week phase of the trial were offered open-label NAC for an additional 6 months. Blinding was maintained until all subjects completed the open-label phase. Drop-out rates and reasons for dropping out of the trial were similar in both arms. Subjects took 3,200–8,000 mg of NAC per day (median, 4,400) for up to 8 months (median, 24 weeks), supplied as 800 mg effervescent tablets by Elan Pharmaceutical (Gainsville, GA).
Assays for GSH.
Total GSH in whole blood was measured in freshly collected blood samples that were mixed within 2 min with 1 ml of 10% sulfosalicylic acid, frozen on dry ice, and stored at −70°C. After thawing, the GSH in the sample was conjugated to monobromobimane and the fluorescent conjugate quantitated by HPLC (24) (modified by M.T.A., unpublished data). Data are expressed as mM GSH by comparison with a GSH standard run with each set of samples.
GSH in PBMC subsets was revealed by using subset-defining antibody combinations to stain cells incubated initially with monochlorobimane, which reacts with intracellular GSH to form GSB, a fluorescent adduct retained by the cells and detectable by FACS using 351 nM excitation. For each PBMC sample, list mode FACS data collected for ≥30,000 events for each of 10 three-color antibody stains were analyzed with facs/desk software developed in our laboratory. Median GSB levels were computed for each PBMC subset from each sample. GSB levels are expressed relative to a reference PBMC aliquot whose median lymphocyte GSB level is defined as one GSB unit.
This GSB FACS assay [refs. 12 and 25, as modified by Anderson et al. (26)] is highly reliable. The interassay coefficient of variation of GSB fluorescences, normalized to the reference aliquot, is <7% for all lymphocyte subsets from a single uninfected individual tested 13 times in 14 months. Similarly, in HIV-infected subjects (≈50), GSB levels measured roughly 1 year after baseline routinely decreased about 10% but otherwise showed little variation. Some of the cell-associated fluorescence measured with this assay may reflect formation of bimane conjugates other than GSB (27); however, the assay’s reliability and the correlation of the GSB values it generates with HPLC-measured GSH in blood (see Fig. 1) validate it as an index of intracellular GSH in PBMC subsets.
Figure 1.
FACS measurement of CD4 T cell GSB fluorescence reflects intracellular GSH levels. GSB is the intracellular fluorescent conjugate of GSH and monochlorobimane. (Top Right) Sample FACS histogram of the GSB fluorescence distribution for CD4 T cells (i.e., GSB in CD4+CD3+ cells) from a single subject. Median (50th percentile) GSB fluorescence levels were computed as shown for each subset in each subject and normalized to the median GSB level for lymphocytes in a PBMC standard analyzed with the samples. Normalized baseline values for the various subsets, labeled as GSB, are shown. (Top Left) This panel relates the median CD4 T cell GSB fluorescence levels to whole blood GSH levels (determined by HPLC) for 53 NAC trial subjects (▪) and 17 male uninfected controls (+). Three HIV-infected outliers (×) were excluded from the analysis (r = 0.56, P < 0.0001 with the outliers included). (Bottom) These panels relate the GSB levels in PBMC subsets from 209 HIV-infected and 82 uninfected subjects. Density ellipses (ovals) include 95% of the points; Pearson correlation coefficient (r) and significance values are computed from a bivariate normal distribution fit (26).
Statistical Analyses.
Macintosh jmp software (SAS Institute, Cary, NC) was used for all statistical analyses (28). Survival curves were compared using the generalized Wilcoxon and Log Rank tests. Wilcoxon P values are shown; Log-Rank P values were always smaller (except where noted). Actuarial tests with covariates were based on Cox Log Rank (proportional hazard) procedures. Logistic regression analyses were based on the survival status of subjects 2–3 years after baseline. Receiver operating characteristic (ROC) analyses were used to define optimal values for discriminating survivors from nonsurvivors in Kaplan–Meier analyses.
RESULTS
FACS GSB Assay Reveals Intracellular GSH.
The multiparameter FACS method used here simultaneously measures the expression of cell surface markers that identify PBMC subsets and, within the cells in the subsets, the level of GSB, the fluorescent conjugate of monochlorobimane and intracellular GSH. Fig. 1 Top Right contains a typical FACS histogram showing the GSB distribution in CD4 T cells from a single individual. The median values for similar GSB distributions, computed individually for each subset in each PBMC sample, are shown in the remaining panels in Fig. 1 and throughout this study.
The correlation between HPLC-measured GSH in whole blood and the FACS-measured GSB in CD4 T cells (r = 0.64, P < 0.0001) justifies this use of GSB fluorescence as a FACS (cell-by-cell) measure of intracellular GSH. This correlation, which is equivalent to the correlation between GSB levels in B cells and CD4 T cells, is actually better than the correlation between GSB levels in CD4 T cells and monocytes (Fig. 1).
GSB Levels Predict Survival.
The hierarchy of baseline CD4 T cell GSB levels (GSB; note that since we report GSB levels in CD4 T cells from this point forward, we use the term GSB exclusively to refer to these levels) for the subject groups in our study is consistent with earlier findings (8–13) (Table 1 and Fig. 2). On average, uninfected subjects have the highest GSB levels; subjects with CD4 T cell counts ≥200/μl have intermediate GSB levels; subjects with CD4 T cell counts <200/μl have lower GSB levels; and, since the NAC trial protocol specified enrollment of GSH-deficient subjects, the subset of our subjects enrolled in the trial have the lowest GSB levels.
Table 1.
CD4 GSB levels are lower in HIV-infected subjects
| Subjects | n* | CD4 GSB†
|
CD4 T cells/μl
|
||
|---|---|---|---|---|---|
| Mean | SD† | Median | IQR† | ||
| Uninfected | 79 | 1.24 | 0.31 | 730‡ | 640–920 |
| All HIV+ | 204 | 0.97 | 0.28 | 209 | 79–371 |
| CD4 T Cells ≥ 200 | |||||
| All | 107 | 1.05 | 0.25 | 356 | 278–480 |
| CD4 T cells < 200§ | |||||
| All | 97 | 0.88 | 0.29 | 72 | 30–129 |
| NoTS cohort | 60 | 0.98 | 0.31 | 74 | 29–128 |
| Trial subjects | 37 | 0.72 | 0.16 | 72 | 43–142 |
Number of subjects for whom survival status was recorded. Overall HIV-infected study group composition: total, 204; male, 198; Caucasian, 155; mean age, 40.4 ± 7.8, range, 23–68.
CD4 GSB values were normally distributed for all groups (Fig. 2). Standard error of the GSB mean (SEM) for the groups shown = 0.02–0.04. IQR, inter-quartile range.
Based on data for 37 subjects.
No trial subjects (NoTS) cohort = all subjects with CD4 T cell counts <200/μl who were not enrolled into the NAC trial but who met the screening criteria (see Methods). Most subjects (39) were not eligible for trial entry because their CD8 GSB levels were too high; the remainder either declined to enter (5 subjects), were judged noncompliant (5 subjects), had a clinical lab value outside the admissible range (7 subjects), or had another protocol-defined exclusion (4 subjects).
Figure 2.
CD4 T cell GSB is lost progressively in HIV disease. Distribution of median baseline GSB levels in CD4 T cells. The vertical bar placed at 1.05 on the GSB axis locates the optimal value (computed by ROC analysis) for discriminating survivors from nonsurvivors among subjects with CD4 counts below 200/μl. Numbers of subjects and statistical data for groups shown are reported in Table 1. See Methods for GSB units; see legend to Table 1 for definition of the NoTS cohort.
We ascertained the survival, 2–3 years later, for all subjects for whom we had baseline FACS data. Because only three subjects with CD4 T cell counts above 200/μl died during this period, we present survival data for all screened subjects and for subjects with CD4 T cell counts below 200/μl (CD4 < 200). Furthermore, because survival patterns for subjects who took NAC were significantly different from the overall cohort (see below), we show data for a cohort of subjects with CD4 < 200 who were not enrolled in the NAC trial—i.e., the NoTS cohort as described in the legend for Table 1.
Kaplan–Meier and logistic regression survival analyses (Figs. 3 and 4) reveal the close relationship between GSB levels and survival. Logistic regression analyses (Fig. 3) show that survival improves as baseline GSB increases. These analyses, which estimate survival at the end of the observation period (2–3 years) as a continuous function of GSB level, show the sharp decrease in the percentage of surviving subjects that occurs at baseline GSB levels that are substantially below normal. Data for the NoTS cohort are particularly dramatic, since subjects in the NAC trial (who are excluded from the cohort) had very low GSB levels but tended to survive longer (see below).
Figure 3.
Survival increases with increasing GSB levels. Logistic regressions in the figure estimate the probability that an individual with a given baseline GSB level (in CD4 T cells) will survive for the entire observation period (2–3 years). None of the subjects showed evidence of debilitating illness at baseline. The regression for the NoTS cohort predicts 65 ± 15% survival for subjects at the mean GSB level for the cohort (0.98); 50% survival for subjects with GSB levels of 0.85 (0.57–1.0, 95% confidence interval); and <40% survival for subjects with GSB levels below 0.72, the mean for subjects in the NAC trial. Histograms show the GSB distributions for survivors (lower histograms) and for nonsurvivors (upper histograms).
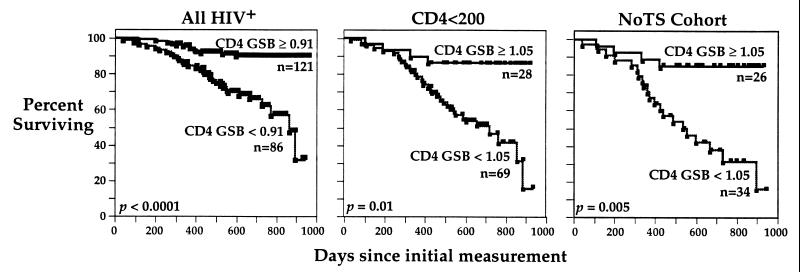
Figure 4.
Low GSB levels predict poor survival. Kaplan–Meier analyses of survival. Subjects are subdivided according to baseline GSB levels (in CD4 T cells); thresholds were determined by ROC analysis. Additional Kaplan–Meier survival analyses (not shown) confirm the previously demonstrated poor prognosis of HIV-infected individuals with low CD4 T cell counts (43 subjects) and hematocrit levels (44 subjects) (P < 0.001 and P = 0.004, respectively, at the optimal threshold computed by ROC for the group with CD4 < 200).
The histograms shown associated with the logistic regressions in Fig. 3 emphasize the difference in GSB levels between survivors (grey bars) and nonsurvivors (filled bars). A simple comparison of the top and bottom quartiles of the overall GSB distributions shows the relationship between GSB and survival: survivors are a distinct minority in the bottom quartiles and an overwhelming majority in the top quartiles (e.g., see Fig. 3).
Kaplan–Meier analyses in Fig. 4 show that subjects with higher baseline GSB levels survive significantly longer than those with lower GSB levels. For these analyses, the optimal threshold for separating survivors from nonsurvivors in each cohort was determined using ROC analyses for subject survival at the end of the 2–3 year study. This threshold, used to group the subjects in each cohort, was the same (1.05) when computed either for the CD4 < 200 cohort (Fig. 4 Center) or the NoTS cohort (Right) and falls at the 30th percentile for uninfected controls (see GSB distribution for controls in Fig. 2). The threshold for all subjects in the study (0.91) is lower and falls at the 20th percentile for uninfected controls.
Proportional hazard analyses in which GSB levels and CD4 T cell counts are included in the model also demonstrate that GSB levels significantly predict survival (Table 2). Although GSB correlates loosely but significantly with CD4 T cell counts (Pearson’s r = 0.33, P < 0.0001), the combined entry of the two parameters in the model confirms the predictive value of GSB independent of its correlation with CD4 T cell count. Thus, by all measures, GSB (in CD4 T cells) emerges as a powerful yardstick for predicting survival in HIV infection. (A comparison with viral load would be very useful; however, viral load data are not available for many of the subjects in this study since most blood samples were collected before methodology for measuring viral load was established.)
Table 2.
CD4 GSB levels predict survival in HIV disease
| HIV-infected subjects | Variable | Risk ratio* | P value† | |
|---|---|---|---|---|
| All | GSB | Continuous | 2.7 (1.8–4.1) | <0.0001 |
| GSB | Continuous | 1.6 (1.1–2.5) | 0.009 | |
| CD4 T cells | Continuous | 1.2 (1.1–1.3) | <0.0001 | |
| CD4 T cells < 200/μl blood | GSB | Continuous | 2.0 (1.3–3.2) | 0.0004 |
| GSB | Continuous | 1.8 (1.2–2.8) | 0.004 | |
| CD4 T cells | Continuous | 1.2 (1.0–1.3) | 0.01 | |
| GSB | Continuous | 2.4 (1.6–3.7) | <0.0001 | |
| NAC | Yes:no | 1.8 (1.2–2.8) | 0.003 | |
| CD4 T cells < 200/μl blood; NoTS cohort | GSB | Continuous | 2.4 (1.5–3.8) | 0.0001 |
Proportional hazard analysis of risk ratio (continuous variables) = increase in probability of surviving per 0.3 GSB unit (GSB standard deviation) or per 20 CD4 T cells/μl blood; 95% confidence limits shown in parentheses.
Effect likelihood calculated for the indicated variables, adjusted for others in the model if it contains more than one variable.
GSH Replenishment Is Associated with Increased Survival.
Survival data for subjects who took NAC provide additional evidence supporting the finding that low GSB levels impair survival. Oral administration of NAC was recently shown to increase plasma cysteine levels in HIV-infected subjects with CD4 T cell counts above 200/μl blood (29). This 4-month study was too short to evaluate survival; however, NAC administration was shown to be associated with a significantly decreased rate of CD4 T cell loss during the trial period.
In our trial, which gave similar results, we measured restoration of whole blood GSH rather than plasma cysteine levels. We found that oral administration of NAC during the 8-week initial (randomized, double-blind, placebo-controlled) phase of the trial largely restored the whole blood GSH levels (Fig. 5; and J.G.D., S.C.D., M.T.A., and L.A.H., unpublished data). After completing this phase of the trial, the majority of subjects in both arms took NAC during a 6-month open-label phase of the trial. We evaluated the survival of these subjects 2 years after initiation of NAC.
Figure 5.
Oral administration of NAC increases whole blood GSH. Whole blood GSH was measured by HPLC. HIV-infected subjects were treated for 8 weeks with orally administered NAC (n = 27) or placebo (n = 26) in a randomized double-blind trial. One very high “outlier” was excluded from the placebo group (P value for comparison at 8 weeks when not excluded = 0.02). Subjects took 3,200–8,000 mg of NAC per day for 8 weeks (median, 4,400 mg). Significance was determined by the Anova t test. The bar in the “means diamond” shows the mean and the vertices show the 95% confidence interval for each group (26). Data for 17 uninfected male controls are shown. A complete report of the trial will be presented elsewhere.
Surprisingly, subjects with CD4 < 200 who took NAC for 8–32 weeks in our study survived significantly longer than a comparable group (also CD4 < 200) who were not offered or did not choose to take NAC (P = 0.002). In essence, the Kaplan–Meier survival curve for the subjects who took NAC is displaced toward higher survival for about the same length of time as the subjects took NAC (Fig. 6). Furthermore, proportional hazard analysis indicates that subjects who took NAC were roughly twice as likely to survive for 2 years as the subjects who did not take NAC (NAC/No-NAC risk ratio = 1.8, 95% confidence interval = 1.1–3.0; P = 0.019).
Figure 6.
Oral administration of NAC is associated with increased survival in AIDS. Data are shown only for subjects with CD4 T cell counts below 200/μl followed for up to 2 years. Subjects took NAC (median, 4,400 mg/day) for 8–32 weeks (median, 24 weeks; interquartile range, 12–27 weeks). Survival times for subjects who took NAC are computed from initiation of NAC administration (0 week for NAC arm; 8 weeks after the trial began for placebo arm). Survival times for subjects who did not take NAC are computed from the trial entry or screening date. All subjects who took NAC were enrolled in the NAC replenishment trial: 13 were randomized to the NAC arm; 12 were randomized to the placebo arm and elected to take open-label NAC during the trial continuation phase. Subjects in the No-NAC group were either enrolled in the NAC trial or met the basic criteria for trial entry (3 completed the placebo arm and declined open-label NAC; 9 left the trial, mainly within a week, citing symptoms such as nausea and rash, which were similar to symptoms reported by subjects who completed the trial; 5 declined to enter the trial for personal reasons; and 2 were disqualified for trial entry only because they had recently changed their reverse transcriptase inhibitor regimen). No significant differences (P > 0.1) at baseline were detected between the NAC and No-NAC groups for the following measurements: absolute counts and GSB levels for CD4 and CD8 naive, memory, and overall T cell subsets, and for B cells, monocytes, and NK cells; hematocrit and other clinical laboratory tests; Karnofsky score; age, weight, and previous opportunistic infections. Of over 60 measurements tested, only plasma thioredoxin levels showed a significant difference between the NAC and No-NAC groups (the NAC group was lower, P = 0.01).
Proportional hazard analyses examining self-selection, compliance, and other issues that could compromise these findings indicate that survival was not affected by the reasons subjects took NAC (randomized to NAC or elected open-label) or by the reasons they did not take NAC (not enrolled in the trial, refused open-label NAC, left the trial). Further, it was not affected by differences in other potentially relevant parameters—e.g., age, weight, Karnofsky score, reverse transcriptase inhibitor usage (P > 0.2 in all cases; see legend for Fig. 6).
The association of oral NAC administration (consequently GSH replenishment) with greater survival is consistent with the dramatically better survival of individuals with higher GSB levels (Fig. 3). However, although the data suggest that NAC ingestion may well contribute to improved survival, we emphasize that no conclusion can be drawn until NAC is administered in a properly controlled prospective clinical trial with survival as the primary endpoint.
DISCUSSION
Findings presented here link GSH deficiency to impaired survival of HIV-infected subjects and suggest a potential intervention to relieve this impairment. In essence, we have shown that GSH levels are lower in subjects with CD4 T cell counts below 200/μl (CD4 < 200) than in subjects at earlier stages of HIV disease; that among subjects with CD4 < 200, lower levels of GSB (a FACS measure of GSH in CD4 T cells) predict decreased survival; and that the probability of surviving 2–3 years increases dramatically as GSB level approach normal range. In addition, we have presented preliminary evidence suggesting that oral administration of NAC, which supplies the cysteine required to replenish GSH, may be associated with improved survival of subjects with very low GSH levels.
The crucial connection revealed here between GSH deficiency and survival in HIV disease was foreshadowed by several studies that demonstrated that HIV-infected people, particularly those with low CD4 T cell counts, often have low levels of GSH in lymphocytes and at other locations (8–14). However, the demonstration here that low baseline GSB levels are associated with decreased survival 2–3 years hence provides the first clear indication that GSH deficiency plays a pivotal role in determining how quickly the final stages of HIV disease progress.
GSH deficiency has long been known to be clinically dangerous in man and in experimental animals. T cell function (16–18, 30–32) and viability (33–35), are markedly impaired in GSH-depleted T cells. Further, AIDS-related cytokines, notably tumor necrosis factor, are particularly toxic when GSH is depleted (15). In fact, tumor necrosis factor will kill experimental animals when administered in high doses unless NAC is rapidly given as an antidote (36). The severe liver and kidney damage caused by exposure to high levels of GSH-depleting drugs such as alcohol and acetaminophen (7, 37) also underscore the dangers of systemic GSH deficiency. Such damage has recently been shown to occur even at relatively low doses of such drugs when systemic GSH levels are compromised (38).
Multiple mechanisms may contribute to systemic GSH deficiency in HIV disease, including excessive production of inflammatory cytokines and excessive use of GSH-depleting drugs. In addition, the HIV infection may itself play a key role through the production and release of HIV-TAT (trans-acting transcriptional activator) (39), since TAT blocks transcription of manganese superoxide dismutase (40–42), an enzyme that helps prevent oxidative stress, and markedly decreases the activity of glucose-6-phosphate dehydrogenase (43), a key enzyme in pathways that maintain GSH in its reduced state.
The preliminary evidence of improved survival associated with oral NAC administration that we report here is consistent both with GSH deficiency being an important determinant of survival in AIDS and with GSH restoration potentially being beneficial. If these findings are confirmed in prospective long-term trials, they will provide the foundation for the use of NAC as an inexpensive, nontoxic adjunct therapy for HIV/AIDS, potentially valuable even in remote locations where only minimal medical supervision is available.
At a more immediate level, the demonstration here that prognosis worsens as GSH levels decrease suggests that certain precautions be taken to minimize GSH deficiency in HIV-infected individuals. In general, it may be prudent for these individuals to avoid excessive exposure to UV irradiation and unnecessary use of drugs that can deplete GSH—e.g., alcohol and prescription or over-the-counter formulations containing acetaminophen.
Acknowledgments
We thank the HIV-infected individuals who generously contributed blood samples, information and sadly, survival data demonstrating how vulnerable we are to HIV. We also thank Dr. B. W. Brown (Stanford University Medical School) and Dr. J. Sall (Senior Vice-President, SAS Institute) for statistical advice. We also thank Dr. F. Staal, Ms. S. O’Leary, Mr. I. Tjioe, Ms. N. Martin, Mr. E. Wunderlich, and other members of our laboratory; the Stanford Center for AIDS Research (directed by Dr. T. Merigan); the Stanford Shared FACS Facility (directed by Dr. D. Parks); Dr. M. Alvarez-Mon (Alcala University, Madrid); Dr. E. Winger and the Immunodiagnostic Laboratory, San Francisco; Davies Medical Center, San Francisco; Drs. S. Wormsley and C.-M. Huang, PharMingen; and Dr. D. Hahn (PharmaQuest, San Rafael, CA). Finally, we thank Dr. C. Myers (University of Virginia) and Dr. S. Broder (IVAC Corporation, Miami, FL) for help in the early stages of this work. The Elan Pharmaceutical Corporation (Gainesville, GA), generously provided NAC and placebo for this study. This work was largely supported by National Institutes of Health grants CA-42509, LM-04836, and 5T32 AI-07290. The Unicorn Foundation (Chicago) and Project Inform (San Francisco) also provided financial support.
ABBREVIATIONS
- GSH
glutathione
- NAC
N-acetylcysteine
- GSB
glutathione-S-bimane fluorescence in CD4 T cells
- PBMC
peripheral blood mononuclear cells
- FACS
fluorescence-activated cell sorter
- ROC
receiver operating characteristic
- NoTS
no trial subjects
Footnotes
A preliminary report was presented at a meeting entitled Oxidative Stress and Redox Regulation in Paris, May 1996.
References
- 1.Vina J, Vinar J R, Saez G T. Life Chem Rep. 1986;4:1–35. [Google Scholar]
- 2.Dolphin D, Avramovic O, Poulson R. Glutathione: Coenzymes and Cofactors. New York: Wiley; 1989. Vols. 3A and 3B. [Google Scholar]
- 3.Taniguchi N, Higashi T, Sakamoto Y, Meister A. Glutathione Centennial. New York: Academic; 1989. [Google Scholar]
- 4.Poot M, Teubert H, Rabinovitch P S, Kavanagh T J. J Cell Physiol. 1995;163:555–560. doi: 10.1002/jcp.1041630316. [DOI] [PubMed] [Google Scholar]
- 5.Adamson D C, Wildemann B, Sasaki M, Glass J D, McArthur J c, Christov V I, Dawson T M, Dawson V L. Science. 1996;274:1917–1919. doi: 10.1126/science.274.5294.1917. [DOI] [PubMed] [Google Scholar]
- 6.Shan X, Aw T Y, Jones D P. Pharmacol Ther. 1990;47:61–71. doi: 10.1016/0163-7258(90)90045-4. [DOI] [PubMed] [Google Scholar]
- 7.Thomas S H. Pharmacol Ther. 1993;60:91–120. doi: 10.1016/0163-7258(93)90023-7. [DOI] [PubMed] [Google Scholar]
- 8.Eck H-P, Gmunder H, Hartmann M, Petzoldt D, Daniel V, Droge W. Biol Chem Hoppe-Seyler. 1989;370:101–108. doi: 10.1515/bchm3.1989.370.1.101. [DOI] [PubMed] [Google Scholar]
- 9.Helbling B, Von Overbeck J, Lauterburg B H. Eur J Clin Invest. 1996;26:38–44. doi: 10.1046/j.1365-2362.1996.88237.x. [DOI] [PubMed] [Google Scholar]
- 10.Buhl R, Holroyd K J, Mastrangeli A, Cantin A M, Jaffe H A, Wells F B, Saltini C, Crystal R G. Lancet. 1989;ii:1294–1298. doi: 10.1016/s0140-6736(89)91909-0. [DOI] [PubMed] [Google Scholar]
- 11.de Quay B, Malinverni R, Lauterburg B H. AIDS. 1992;6:815–819. [PubMed] [Google Scholar]
- 12.Roederer M, Staal F J T, Osada H, Herzenberg L A, Herzenberg L A. Int Immunol. 1991;3:933–937. doi: 10.1093/intimm/3.9.933. [DOI] [PubMed] [Google Scholar]
- 13.Staal F J T, Roederer M, Israelski D M, Bubp J, Mole L A, McShane D, Deresinski S C, Ross W, Sussman H, Raju P A, Anderson M T, Moore W, Ela S W, Herzenberg L A, Herzenberg L A. AIDS Res Human Retroviruses. 1992;8:305–314. doi: 10.1089/aid.1992.8.305. [DOI] [PubMed] [Google Scholar]
- 14.Staal F J T, Ela S W, Roederer R, Anderson M T, Herzenberg L A, Herzenberg L A. Lancet. 1992;339:909–912. doi: 10.1016/0140-6736(92)90939-z. [DOI] [PubMed] [Google Scholar]
- 15.Adamson G M, Billings R E. Arch Biochem Biophys. 1992;294:223–229. doi: 10.1016/0003-9861(92)90161-o. [DOI] [PubMed] [Google Scholar]
- 16.Staal F J T, Anderson M T, Staal G E J, Herzenberg L A, Gitler C, Herzenberg L A. Proc Natl Acad Sci USA. 1994;91:3619–3622. doi: 10.1073/pnas.91.9.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Staal F J T, Roederer M, Herzenberg L A, Herzenberg L A. Proc Natl Acad Sci USA. 1990;87:9943–9947. doi: 10.1073/pnas.87.24.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mihm S, Ennen J, Pessara U, Kurth R, Droge W. AIDS. 1991;5:497–503. doi: 10.1097/00002030-199105000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez A, Kiefer J, Fosdick L, McConkey D J. J Immunol. 1995;155:5133–5139. [PubMed] [Google Scholar]
- 20.Bagasra O, Bachman S E, Jew L, Tawadros R, Cater J, Boden G, Ryan I, Pomerantz R J. J Infect Dis. 1996;173:550–558. doi: 10.1093/infdis/173.3.550. [DOI] [PubMed] [Google Scholar]
- 21.Stanley S K, Folks T M, Fauci A S. AIDS Res Human Retroviruses. 1989;5:375–383. doi: 10.1089/aid.1989.5.375. [DOI] [PubMed] [Google Scholar]
- 22.Stein B, Rahmsdorf H J, Steffen A, Litfin M, Herrlich P. Mol Cell Biol. 1989;9:5169–5191. doi: 10.1128/mcb.9.11.5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vincek V. Med Hypotheses. 1995;44:119–123. doi: 10.1016/0306-9877(95)90083-7. [DOI] [PubMed] [Google Scholar]
- 24.Anderson M E. Methods Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- 25.Roederer M, Staal F J T, Anderson M, Rabin R, Raju P A, Herzenberg L A, Herzenberg L A. Ann NY Acad Sci. 1993;677:113–125. doi: 10.1111/j.1749-6632.1993.tb38770.x. [DOI] [PubMed] [Google Scholar]
- 26.Anderson, M. T., Roederer, M., Tjioe, I., Herzenberg, L. A. & Herzenberg, L. A., (1996) in The Handbook of Experimental Immunology, eds. Herzenberg, L. A., Herzenberg, L. A., Blackwell, C. & Weir, D. (Blackwell Scientific, Boston), 5th Ed., Vol. 1B, Chap. 54 pp. 1–9.
- 27.Hedley D W, Chow S. Cytometry. 1994;14:349–358. doi: 10.1002/cyto.990150411. [DOI] [PubMed] [Google Scholar]
- 28.JMP Development Group. JMP Statistics and Graphics Guide, Version 3.1. Cary, NC: SAS Institute; 1995. [Google Scholar]
- 29.Akerlund B, Jarstrand C, Lindeke B, Sonnerborg A, Akerblad A C, Rasool O. Eur J Clin Pharmacol. 1996;50:457–461. doi: 10.1007/s002280050140. [DOI] [PubMed] [Google Scholar]
- 30.Roederer M, Staal F J T, Raju P A, Ela S W, Herzenberg L A, Herzenberg L A. Proc Acad Natl Sci USA. 1990;87:4884–4888. doi: 10.1073/pnas.87.12.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eylar, E. H., Baez, I., Vazquez, A. & Yamamura, Y. (1995) Cell. Mol. Biol. 41, Suppl. 1, S35–S40. [PubMed]
- 32.Jeannin P, Delneste Y, Lecoanet-Henchoz S, Gauchat J-F, Life P, Holmes D, Bonnefoy J-Y. J Exp Med. 1995;182:1785–1792. doi: 10.1084/jem.182.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandstrom P A, Mannie M D, Buttke T M. J Leukocyte Biol. 1994;55:221–226. doi: 10.1002/jlb.55.2.221. [DOI] [PubMed] [Google Scholar]
- 34.Mayer M, Noble M. Proc Natl Acad Sci USA. 1994;91:7496–7500. doi: 10.1073/pnas.91.16.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olivier R, Lopez O, Molereau M, Dragic T, Guetard D, Montagnier L. In: Oxidative Stress, Cell Activation and Viral Infection. Pasquier C, Olivier R Y, Auclair C, Packer L, editors. Boston: Birkhauser; 1994. pp. 323–332. [Google Scholar]
- 36.Zimmerman R J, Marafino B J, Jr, Chan A, Landre P, Winkelhake J L. J Immunol. 1989;142:1405–1409. [PubMed] [Google Scholar]
- 37.Bondy S C. Toxicol Lett. 1992;63:231–241. doi: 10.1016/0378-4274(92)90086-y. [DOI] [PubMed] [Google Scholar]
- 38.Zimmerman H J, Maddrey W C. Hepatology. 1995;22:767–773. [PubMed] [Google Scholar]
- 39.Ehret A, Westendorp M O, Herr I, Debatin K M, Heeney J L, Frank R, Krammer P H. J Virol. 1996;70:6502–6507. doi: 10.1128/jvi.70.9.6502-6507.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flores S C, Marecki J C, Harper K P, Bose S K, Nelson S K, McCord J M. Proc Natl Acad Sci USA. 1993;90:7632–7636. doi: 10.1073/pnas.90.16.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCord J, Flores S. In: Oxidative Processes and Antioxidants. Paoletti R, editor. New York: Raven; 1994. pp. 13–23. [Google Scholar]
- 42.Westendorp M O, Shatrov V A, Schulze-Osthoff K, Frank R, Kraft M, Los M, Krammer P H, Droge W, Lehmann V. EMBO J. 1995;14:546–554. doi: 10.1002/j.1460-2075.1995.tb07030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flores, S. & McCord, J., in Oxyradicals in Medical Biology, ed. McCord, J. (JAI, Greenwich, CT), in press.