Abstract
Phototransduction in retinal rods involves a G protein-coupled signaling cascade that leads to cGMP hydrolysis and the closure of cGMP-gated cation channels that are open in darkness, producing a membrane hyperpolarization as the light response. For many years there have also been reports of the presence of a phosphoinositide pathway in the rod outer segment, though its functions and the molecular identities of its components are still unclear. Using immunocytochemistry with antibodies against various phosphoinositide-specific phospholipase C (PLC) isozymes (β1–4, γ1–2, and δ1–2), we have found PLCβ4-like immunoreactivity in rod outer segments. Similar experiments with antibodies against the α-subunits of the Gq family of G proteins, which are known to activate PLCβ4, have also demonstrated Gα11-like immunoreactivity in this location. Immunoblots of total proteins from whole retina or partially purified rod outer segments with anti-PLCβ4 and anti-Gα11 antibodies gave, respectively, a single protein band of the expected molecular mass, suggesting specific labelings. The retinal locations of the two proteins were also supported by in situ hybridization experiments on mouse retina with probes specific for the corresponding mouse genes. These two proteins, or immunologically identical isoforms, therefore likely mediate the phosphoinositide signaling pathway in the rod outer segment. At present, Gα11 or a Gα11-like protein represents the only G protein besides transducin (which mediates phototransduction) identified so far in the rod outer segment. Although absent in the outer segment layer, other PLC isoforms as well as Gαq (another Gq family member), are present elsewhere in the retina.
Keywords: phospholipase C, G protein, visual transduction
Visual transduction in the retina takes place in the outer segments of rod and cone photoreceptors and is known to involve a cGMP signaling cascade (see ref. 1 for a recent review). In this process, light isomerizes the visual pigment into an active form, which, via the G protein transducin, stimulates a cGMP-phosphodiesterase to lead to cGMP hydrolysis. In darkness, cytoplasmic cGMP binds to and opens cGMP-activated cation channels on the plasma membrane of the outer segment. These open channels sustain an inward dark current to keep the cell partially depolarized and maintain a steady release of glutamate from the synaptic terminal of the photoreceptor. In the light, the hydrolysis of cGMP leads to the closure of these channels, producing a membrane hyperpolarization as the light response and reducing the glutamate release from the cell.
Over the years, however, there have also been reports of a phosphoinositide signaling pathway in the rod outer segment. In this pathway, the membrane phospholipid phosphatidylinositol-4,5-bisphosphate is hydrolyzed by phospholipase C (PLC) enzymes to release the second messengers inositol-1,4,5-trisphosphate and diacylglycerol, with the former leading to intracellular Ca2+ release and the latter activating the enzyme protein kinase C (PKC). In rod outer segment preparations, phosphoinositide-specific PLC activity has been observed (2–7) and reported to be activated by light (2–5). This enzyme in the rod outer segment has also been characterized biochemically (6–8) and immunologically (8). Finally, PKC activity in the same location has been reported (9–12), with putative substrates including rhodopsin (9, 13–15), the α-subunit of transducin (16), the inhibitory γ-subunit of the cGMP phosphodiesterase (17–19), guanylate cyclase (20), and arrestin (21), all of which are involved in rod phototransduction. Despite these findings, the identities of the components of the phosphoinositide pathway have remained unknown. To address this question, we have undertaken an immunocytochemical approach, using specific antibodies directed against different PLC isoforms. As presently known, these enzymes can be divided according to their amino acid sequences and functional properties into three families, designated β, γ, and δ (see refs. 22–24 for recent reviews). The PLCβ family is coupled to seven-transmembrane-helix receptors through the α-subunits of the pertussis toxin-insensitive Gq family of G proteins and/or through G protein βγ-subunits. The PLCγ family, on the other hand, is activated by growth factor receptors via tyrosine phosphorylation. The activation mechanism for the PLCδ family remains unknown. In this study, we have found that, even though almost all of the PLC isozymes examined are in the retina, only PLCβ4 is present in the rod outer segment. This has led us also to examine several α-subunits of the Gq family of G proteins for colocalization in the rod outer segment. Only Gα11-like immunoreactivity was found in this location. These findings have previously been reported in abstract form (25).
METHODS
Antibodies.
The anti-PLCβ1, β2, β3, β4, γ2, and δ2 antibodies are rabbit polyclonal antisera, and the anti-PLCγ1 and δ1 antibodies are mouse monoclonal antibodies. Two anti-PLCβ4 antibodies were used and gave identical results in the immunocytochemistry and immunoblotting experiments described here. Of the two, the first (Ab1) is against purified bovine retinal PLCβ4 (26), and the second (Ab2) is a mixture of two antibodies against, respectively, synthetic peptides (AVFDRYEEESFV and VKLEAEMDRRPATV) based on the N and C termini of the cloned rat PLCβ4 protein (27). In immunoblots, both Ab1 and Ab2 (as an example, see Fig. 1 for Ab2) clearly recognized the purified PLCβ4 protein from bovine retina as well as HPLC fractions from rat uterus that showed high PLC activity. All other antibodies are against the respective bovine proteins. The characterization of these antibodies has been described elsewhere (26–29). Three rabbit polyclonal antibodies against several α-subunits of the Gq family of G proteins were used, kindly provided by John H. Exton (Vanderbilt University, Nashville, TN). These antipeptide antibodies, designated E973, E976, and 24, recognize Gαq, Gα11, and Gα14, respectively; the specificities of two of these antibodies (E973, E976) have been described (30, 31). Finally, the antibody PMc1D1, kindly provided by Robert S. Molday (University of British Columbia, Vancouver) and directed against the rod cGMP-gated channel (32), was used to check the degree of purity of partially purified rod outer segment preparations (see Results).
Figure 1.
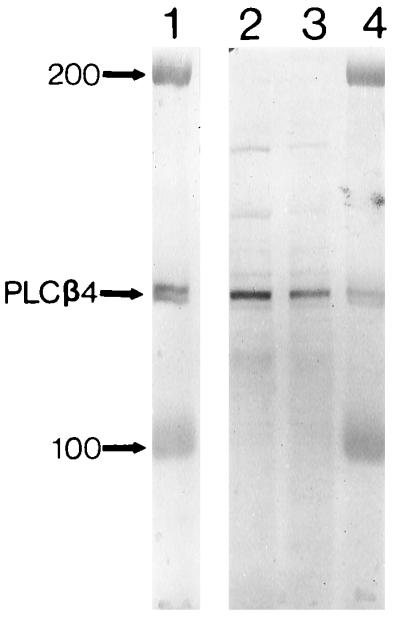
Immunoblotting of purified PLCβ4 from bovine retina (lanes 1 and 4) and HPLC fractions from rat uterus that show high phospholipase C activity (lanes 2 and 3), using the Ab2 antibody. The numbers indicate protein markers of molecular masses 100 and 200 kDa. See refs. 26 and 27 for the methods of collecting the HPLC fractions and assaying phospholipase C activity.
Immunocytochemistry.
All immunocytochemistry was performed on frozen retinal sections prefixed with 4% paraformaldehyde (33). The described results are for bovine retina, but similar results were obtained from rat, mouse, and rabbit retinas. For immunoperoxidase staining, the sections were first incubated with 5% normal goat serum (Vector Laboratories) in PBS for 1 hr at room temperature to reduce background staining. The sections were then incubated overnight at 4°C with the primary antibody (1:10,000 dilution for anti-PLCβ1, 1:2000 for anti-PLCβ2, 1:3000 for anti-PLCβ3, 1:2000 for anti-PLCβ4, 1:500 for anti-PLCγ1, 1:5000 for anti-PLCγ2, 1:400 for anti-PLCδ1, and 1:2000 for anti-PLCδ2, Gαq, and Gα11; no specific staining with anti-Gα14 was found). This was followed by two washes in PBS, each for 30 min. Triton X-100 (0.3%) was added to all incubation and wash buffers. Depending on the primary antibody, the sections were then incubated with a biotin-conjugated goat anti-rabbit or anti-mouse secondary antibody (Vector Laboratories; 1:200 dilution) for 2 hr at room temperature, then washed twice in PBS for 30 min each, followed by a 1-hr incubation with an avidin-biotin-peroxidase complex (Vector Laboratories; 1:100 dilution) in PBS. After two more washes for 30 min each, the stain was developed with a substrate solution of 20 ml PBS, 0.1 ml 3% H2O2, and 10 mg diaminobenzidine. The staining reaction was terminated by washing with PBS, and the sections were coverslipped with 50% glycerol in PBS. For each antibody, the specificity of staining was also confirmed by preadsorbing the corresponding antigen to the antibody, which resulted in no staining.
Immunoblotting.
Immunoblotting experiments on total proteins from whole bovine retina or partially purified bovine rod outer segments were carried out with the antibodies against PLCβ4, Gα11, Gαq, and PLCγ2, as well as PMc1D1. For total proteins, a bovine retina was homogenized in 5% SDS, 1 mM benzamidine, 0.5 mM phenylmethylsulfonyl fluoride, and 1 mM EDTA in Tris-buffered saline (TBS). Insoluble material was removed by centrifugation at 13,000 × g for 10 min. Proteins were assayed using Pierce reagent. SDS/PAGE was performed on 1.5-mm thick, 5–16% polyacrylamide gels, and the separated proteins were transferred to nitrocellulose membranes (34). The blots were blocked with 5% nonfat dry milk in TBS for 2 hr before an overnight incubation at 4°C with the primary antibodies diluted in 3% BSA in TBS (1:2000 for all three antibodies). Afterwards, they were washed three times for 10 min each in 5% nonfat dry milk in TBS, then incubated with a horseradish peroxidase-linked, goat anti-rabbit or anti-mouse secondary antibody for 1 hr at room temperature. After three washes of 10 min each in TBS, the blots were developed using 4-chloro-1-naphthol as the substrate. Proteins from partially purified rod outer segments were treated in a similar way. The method for purifying rod outer segments has been described elsewhere (35).
In situ Hybridization.
Frozen sections of paraformaldehyde-fixed mouse retina were prepared as described above, but with standard procedures to eliminate RNase activity. The sections were rinsed in 2× standard saline citrate (SSC; 1× SSC = 0.15 M sodium chloride/0.015 M sodium citrate, pH 7) and incubated in 2–10 mg/ml proteinase K in Tris·EDTA for 15–20 min at 37°C. The slides were then washed in 2× SSC and treated with 0.1 M triethanolamine/0.25% acetic anhydride. The sections were then covered with a sense or antisense RNA probe diluted to 1 mg/ml in hybridization buffer, coverslipped, and sealed with nail polish. These sealed slides were incubated overnight at 50°C. The coverslips were then removed and the slides washed in 2× SSC. After the sections were incubated in 20 mg/ml RNase A in RNase buffer for 30 min, the slides were washed successively in 2× SSC, 1× SSC, and 0.1× SSC at 50°C. After washing at room temperature in PBS/0.1% Tween 20, the sections were blocked in 5% normal goat serum. An anti-digoxygenin antibody conjugated with alkaline phosphatase (Boehringer Mannheim, 1:500 diluted in PBS) was then added to the slides and incubated overnight. The slides were developed for 2–4 hr with color reaction and sealed with coverslips.
To prepare RNA probes for in situ hybridization, PCRs were performed using mouse brain cDNA as template to obtain fragments corresponding to published sequences for nucleotides 441–763 of mouse Gα11 and 313–622 of rat PLCβ4. The PCR products were then subcloned into a TA cloning vector (pCR II; Invitrogen). The resulting plasmids showed 100% and 97% identities, respectively, to published mouse Gα11 and rat PLCβ4 sequences. Digoxygenin-labeled RNA probes, sense or anti-sense, were made with a commercial kit (Boehringer Mannheim).
Dissociated Cells.
The isolated bovine retina was incubated at room temperature in DMEM (GIBCO) supplemented with 10 units/ml papain (Worthington), 1.2 mM EDTA, and 5.5 mM cysteine. After 45 min of incubation, the retina was washed with DMEM containing bovine serum albumin (0.1 mg/ml). Dissociation of the treated retina into individual cells was then effected by gentle trituration with a wide-bore transfer pipette. Aliquots of freshly dissociated cells were placed in a test tube and fixed for 2 hr with 4% paraformaldehyde in phosphate buffer at 4°C. The fixed cells were pipetted onto poly-d-lysine-coated slides and left to settle for 2 hr. The subsequent immunostaining procedures were identical to those described for retinal sections.
RESULTS
PLCβ4- and Gα11-like Immunoreactivities in Rod Outer Segments.
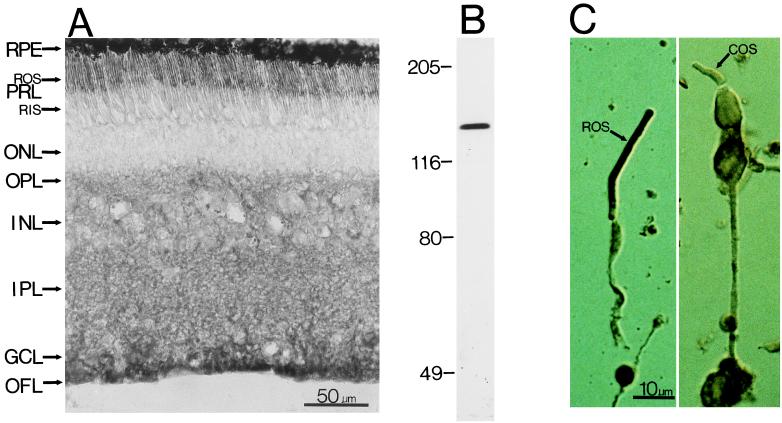
Among all of the PLC isozymes we have examined (PLCβ1–4, γ1–2, and δ1–2), only PLCβ4-like immunoreactivity is present in the photoreceptor outer segment layer. Fig. 2A shows the staining in a bovine retinal section. Examination of dissociated photoreceptors indicates that only rod outer segments, but apparently not cone outer segments, are stained (Fig. 2C). PLCβ4-like staining is also present in the outer plexiform layer and the inner retina. The retinal staining seems specific, because, besides preadsorption experiments as negative controls (see Methods), immunoblotting of total proteins from bovine retina gave a single band of molecular mass (≈130 kDa) appropriate for PLCβ4 (Fig. 2B).
Figure 2.
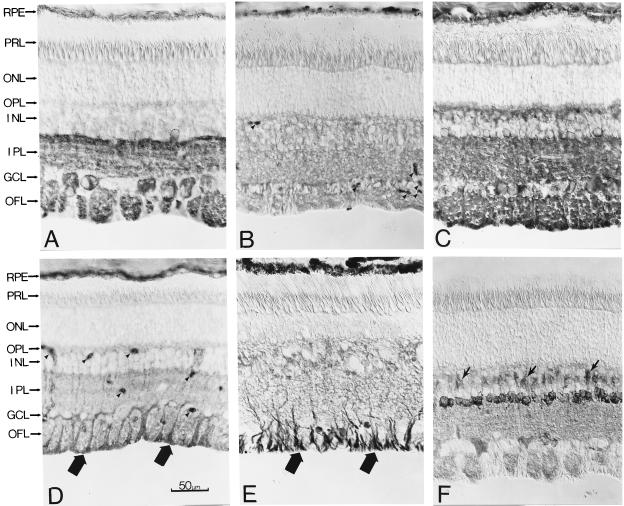
(A) Immunostaining of a cross-section of the bovine retina with an antibody against PLCβ4. (Nomarski differential interference contrast optics; 8-μM frozen section.) The anatomical layers are as follows: RPE, retinal pigment epithelium; PRL, photoreceptor layer containing predominantly the outer segments (ROS) and inner segments (RIS) of rods, though cones are also present in far fewer numbers; ONL, outer nuclear layer containing the cell bodies of the photoreceptors; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer; OFL, optic fiber layer. (B) Immunoblotting of total bovine retinal proteins with the anti-PLCβ4 antibody. Sizes of molecular mass standards (×10−3) are shown adjacent to the lane. (C) Dissociated rod (Left) and cone (Right) cells stained with the same antibody. Only the rod outer segment (ROS) shows staining, but apparently not the cone outer segment (COS).
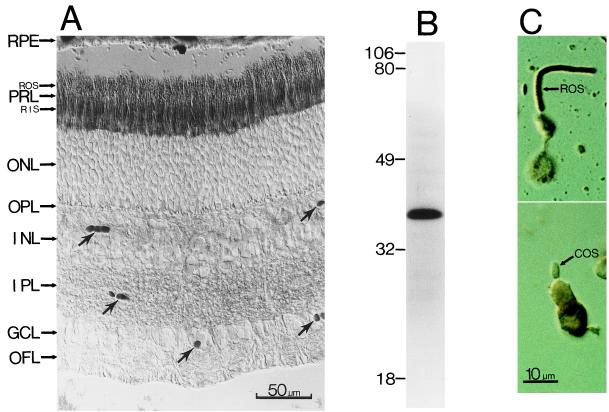
Because PLCβ4 has been shown to be activated by the α-subunits of the Gq family of G proteins (36, 37), we have examined the distributions of some of these α-subunits (Gαq, Gα11, and Gα14) in the retina as well. Among these, only Gα11-like immunoreactivity was found in rod outer segments. Fig. 3A shows this staining in bovine retina. It is present mostly in the outer and inner segment layers (stained structures in the inner retina marked by arrows are blood vessels); again, dissociated-cell studies indicate that only rod outer segments, but apparently not cone outer segments, show staining (Fig. 3C). Immunoblotting of total proteins from bovine retinas with the anti-Gα11 antibody likewise gave a single protein band of molecular mass (≈42 kDa) appropriate for this G protein, suggesting specific labeling (Fig. 3B).
Figure 3.
(A) Immunostaining of a cross-section of the bovine retina with an antibody against Gα11. (Nomarski optics; 8-μM frozen section.) The anatomical layers are as in Fig. 2. The arrows indicate blood vessels. (B) Immunoblotting of total bovine retinal proteins with the anti-Gα11 antibody. Sizes of molecular mass standards (×10−3) are shown adjacent to the lane. (C) Dissociated rod (Top) and cone (Bottom) cells stained with the same antibody. Again, only the rod outer segment (ROS), but apparently not the cone outer segment (COS), shows staining.
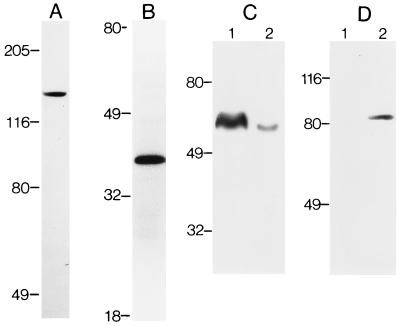
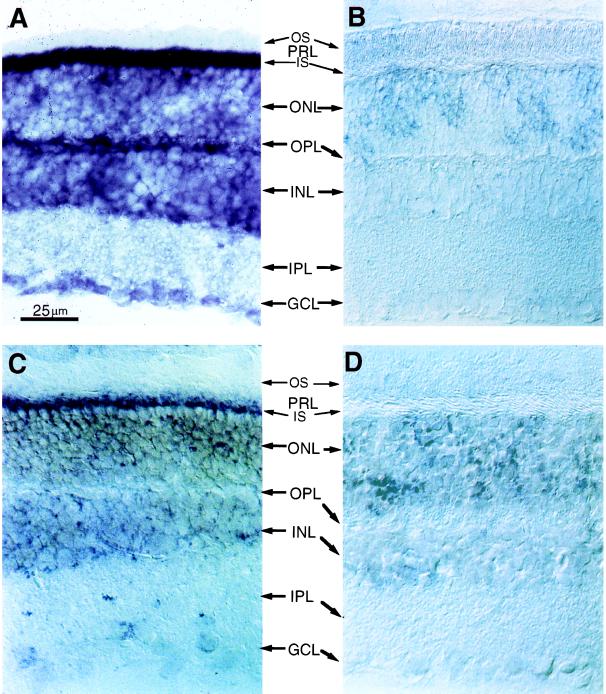
Two other kinds of experiments were carried out to confirm the presence of PLCβ4 and Gα11 in rod outer segments. First, we performed immunoblotting of proteins from partially purified rod outer segment preparations (Fig. 4) with the anti-PLCβ4 and anti-Gα11 antibodies. In each case, a single protein band of appropriate molecular mass was observed (Fig. 4 A and B). Labelings with an antibody against the rod cGMP-gated channel (which is present almost exclusively in the rod outer segment) and the antibody against PLCδ2 (which is absent in the rod outer segment; see below) indicated that the partially purified rod outer segment preparations we used were indeed concentrated with rod outer segment proteins (Fig. 4 C and D). Second, in situ hybridizations were performed on mouse retinal sections using anti-sense cRNA probes specific for mouse PLCβ4 and Gα11 mRNA (see Methods). The mouse was chosen because nucleotide sequence information was available in the database for Gα11 in this species and for PLCβ4 in the closely related rat. For PLCβ4, the hybridization signal is present in the inner segment layer (where most photoreceptor mRNA is situated) and the outer nuclear layer (which contains the cell bodies of photoreceptors), as well as cell bodies in the inner nuclear layer and the ganglion cell layer (Fig. 5A). The intense signal in the photoreceptors is thus consistent with the immunocytochemical results. The hybridization in the inner nuclear layer and the ganglion cell layer likewise agrees with the immunostaining in the inner retina. For Gα11, the hybridization signal is confined mostly to the inner segment layer (Fig. 5C) and is also in agreement with the immunocytochemistry. Control experiments with sense cRNA probes did not give any staining (Fig. 5 B and D).
Figure 4.
Immunoblotting of total proteins from partially purified bovine rod outer segments. (A) With the anti-PLCβ4 antibody. (B) With the anti-Gα11 antibody. (C) Experiment examining the degree of purity of the partially purified rod outer segment preparation. Lane 1, partially purified rod outer segments; lane 2, total retinal proteins. Both are stained with the PMc1D1 antibody against the rod cGMP-gated channel, known to be present predominantly in the rod outer segment (38). Densitometric measurements indicated that the intensity of staining was approximately 10:1 between lanes 1 and 2. (D) Experiment and display similar to C, but stained with an antibody against PLCδ2, which is predominantly in Müller cells rather than rod outer segments (see Results). Sizes of molecular mass standards (×10−3) are shown adjacent to the lanes.
Figure 5.
In situ hybridization of mouse retinal sections with digoxygenin-labeled riboprobes. The anatomical layers are as in Fig. 2. (A) Bright-field optics; with antisense riboprobe for PLCβ4. (B–D) Nomarski optics; 8-μM frozen sections. (B) With sense riboprobe for PLCβ4. (C) With antisense riboprobe for Gα11. (D) With sense riboprobe for Gα11.
Other PLC Isozymes and Gq Family Members in the Retina.
Although PLCβ4 is the only isoform present in the outer segment layer, we have found other isoforms elsewhere in the retina. For PLCβ1, the immunoreactivity is confined mostly to the inner plexiform layer, some amacrine and ganglion cells, and the optic fiber layer (Fig. 6A). No PLCβ2 immunostaining was observed, which is in agreement with previous immunoblotting results (28). Finally, PLCβ3 staining has a weak, diffuse appearance in the bovine retina, spanning the outer plexiform layer and more proximal layers (Fig. 6B). As with PLCβ4, PLCβ1 and β3 are activated by the α-subunits of the Gq family of G proteins (22–24). It is thus relevant that their locations in the inner retina are roughly coincident with that of Gαq, another Gq family member present in the retina (see below). PLCβ3, and to a much lesser degree PLCβ1, can also be activated by G protein βγ-subunits (23, 24), but in this study we have not pursued this point further in the retina.
Figure 6.
Immunostaining of bovine retinal cross-sections for other PLC isozymes and also Gαq. (Nomarski optics; 8-μM frozen sections.) The anatomical layers are as in Fig. 2. (A) PLCβ1. (B) PLCβ3. (C) PLCγ1. (D) PLCδ1 (thick arrows indicate stained endfeet of Müller glial cells; arrowheads indicate blood vessels). (E) PLCδ2 (arrows indicate stained endfeet of Müller glial cells). (F) Gαq (arrows indicate stained bipolar cells).
For the PLCγ isoforms examined, only PLCγ1 staining was detected. The staining is even more intense and widespread than that of PLCβ4, but is absent in rod outer segments (Fig. 6C). In the brain, this enzyme is also widespread (39, 40). Experiments with dissociated cells have confirmed staining in different retinal cell types, including photoreceptors, though the photoreceptor staining is confined to synaptic terminals, which are situated in the outer plexiform layer (data not shown). The PLCγ isoforms are known to be activated by growth-factor receptors through tyrosine phosphorylation (22–24). Such receptors are widespread in the retina (e.g., see ref. 41).
The antibodies against PLCδ1 and PLCδ2 both stained Müller glial cells exclusively, in particular their endfeet regions (Fig. 6 D and E, large arrows; in Fig. 6D, small arrowheads indicate blood vessels). In the brain, these enzyme isoforms are also present predominantly in glial cells (42, 43). Their specific associations with glial cells may imply some unique functions.
As for other members of the Gq family, we have found Gαq but not Gα14 immunoreactivity in the retina. The Gαq immunostaining is not in photoreceptors but is primarily in the inner nuclear layer (Fig. 6F). The stained cell bodies in the latter location appear to be predominantly amacrine cells, though some bipolar cells (arrows) and some ganglion cells also show staining. Despite their high homology to each other and colocalizations in most tissues (44–46), recent work has suggested that Gα11 and Gαq can have differential functions, by, for example, coupling to different receptors (47). Our finding that the two proteins have differential localizations in the retina supports this point.
Overall, the combined distribution of all of the PLC isozymes we have examined partially match that of the IP3 receptor we have found previously, which is predominantly in the two plexiform layers and Müller glial cells (33). One prominent difference is the absence of IP3 receptor staining in the photoreceptor outer segment layer (33, 48). Minor differences also exist in the inner retina. It is possible that the antibody used in our previous study does not recognize all forms of the IP3 receptor. Another possibility is that there is a functional bias toward the PKC branch instead of the IP3 branch of the phosphoinositide pathway in certain cell locations, such as the case in the rod outer segment.
DISCUSSION
The existence of a phosphoinositide signaling pathway in the outer segments of vertebrate photoreceptor cells has been a topic of interest for many years, though the molecular identities of its components remained unknown. Our results suggest that PLCβ4 and Gα11, or immunologically identical isoforms, may be the constituents of this pathway. The amino acid sequences of the cloned bovine and rat PLCβ4 (49, 50) suggest that it is the mammalian homolog of a Drosophila PLC enzyme present predominantly in the eyes (51). A defect in this Drosophila enzyme results in a mutant called norpA, the photoreceptors of which fail to give any electrical response to light (52, 53); thus, this protein, called PLC-norpA, appears to be crucial for invertebrate phototransduction. The phototransduction pathways in Drosophila or other invertebrate rhabdomeric photoreceptors, nonetheless, are still unclear. By contrast, the central role of a cGMP cascade in phototransduction is well established in vertebrate rods (see Introduction), though Ca2+ also exerts an important negative-feedback control on this pathway. Thus, in rods, the phosphoinositide pathway can, in principle, interact with the cGMP cascade through IP3 production and regulation of intracelluar Ca2+. So far, however, the evidence for such a mechanism is largely lacking. For example, the observed light-triggered production of IP3 in rod outer segments is very transient (4, 5); in fact, there is no uniform agreement as to whether light actually influences phosphoinositide turnover (6, 7). So far, there also is no evidence for the presence of an IP3 receptor in the rod outer segment, as pointed out in Results. Finally, the present understanding of rod phototransduction, which is quite detailed, does not need to invoke any IP3-mediated Ca2+ mobilization (1). Taken together, these points suggest that the IP3 branch of the phosphoinositide pathway may be insignificant in the rod outer segment, at least with respect to its canonical mode of function (i.e., via an IP3 receptor). The evidence for a function of the diacylglycerol-PKC branch of the pathway, on the other hand, is more substantial (9–21). In particular, its proposed role in light adaptation through phosphorylation of both bleached and unbleached rhodopsin (13–15) is intriguing, though confirmation from physiological experiments has yet to be made. There is also the possibility that the phosphoinositide pathway is involved in functions other than phototransduction in the outer segment, and triggered by a chemical signal rather than light.
A discrepancy between our study and a previous study by Ferreira and Pak (54) should be mentioned here. Using antibodies different from ours against PLCβ4, these investigators have localized the enzyme to cones and not rods. The reason for this discrepancy is presently unclear, especially because earlier in situ hybridization experiments by the same group (49) have identified substantial message in the outer nuclear layer of the rod-dominant bovine retina, a result seemingly consistent with our present finding.
The colocalizations of PLCβ4- and Gα11-like immunoreactivities in the rod outer segment agree with the biochemical finding that PLCβ4 is specifically activated by α-subunits of the Gq family but not by rod transducin (28, 36, 37). Incidentally, Gα11 (or a very homologous isoform) is at present the only G protein besides transducin identified in the rod outer segment. An obvious question is whether Gα11 bears any functional relation to transducin in this location, and which Gβγ subunits Gα11 is coupled to. Rod transducin (Gαt1) is known to be coupled to Gβ1 and Gγ1, the only G protein β- and γ-subunits so far identified in rod outer segments (55). Thus, it will be interesting to know whether the same or different β- and γ-subunits are involved in the function of Gα11. Another issue has to do with the apparent absence of PLCβ4 (at least according to our results) and Gα11 stainings in cone outer segments. Given its presence in rods, the phosphoinositide pathway is most likely present in cones as well, thus raising questions about the identities of the corresponding PLC enzyme and Gα protein in cones. One G protein β-subunit (Gβ3), but two γ-subunits (Gγ2 and Gγ8), have recently been identified in cone outer segments (55–57). Biochemical analysis suggests that, functionally, Gγ8 is likely the cone homolog of rod Gγ1 and coupled to cone transducin (Gαt2) (57). Perhaps, then, Gγ2 is coupled to the yet-to-be-identified Gq member in cone outer segments. Two α-subunits of the Gq family not examined in our study are Gα15 and Gα16, but these two proteins are thought to be predominantly in hematopoietic tissues (58, 59). Possibly, a novel Gq family member exists. The same applies to the PLC isoform in cone outer segments. It is interesting that distinct molecular species seem to exist in rods and cones for many proteins of known function.
Acknowledgments
We thank Dr. S. B. Lee for providing the data in Fig. 1 and Dr. Y. S. Bae for preparing the figure. We also thank Drs. John H. Exton and Robert S. Molday for kindly providing us with their antibodies, and Dr. Tom Wilkie for comments on the manuscript. This work was supported by National Institutes of Health grant EY06837 to K.-W. Yau.
ABBREVIATION
- PLC
phospholipase C
References
- 1.Yau K-W. Invest Ophthalmol Visual Sci. 1993;35:9–32. [PubMed] [Google Scholar]
- 2.Ghalayini A J, Anderson R E. Biochem Biophys Res Commun. 1984;124:503–506. doi: 10.1016/0006-291x(84)91582-1. [DOI] [PubMed] [Google Scholar]
- 3.Hayashi F, Amakawa T. Biochem Biophys Res Commun. 1985;128:954–959. doi: 10.1016/0006-291x(85)90139-1. [DOI] [PubMed] [Google Scholar]
- 4.Brown J E, Blazynski C, Cohen A I. Biochem Biophys Res Comm. 1987;146:1392–1396. doi: 10.1016/0006-291x(87)90804-7. [DOI] [PubMed] [Google Scholar]
- 5.Millar F A, Fisher S C, Muir C A, Edwards E, Hawthorne J N. Biochim Biophys Acta. 1988;970:205–211. doi: 10.1016/0167-4889(88)90180-2. [DOI] [PubMed] [Google Scholar]
- 6.Gehm B D, McConnell D G. Biochemistry. 1990;29:5447–5452. doi: 10.1021/bi00475a006. [DOI] [PubMed] [Google Scholar]
- 7.Panfoli I, Morelli A, Pepe I. Biochem Biophys Res Commun. 1990;173:283–288. doi: 10.1016/s0006-291x(05)81054-x. [DOI] [PubMed] [Google Scholar]
- 8.Ghalayini A J, Tarver A P, Mackin W M, Koutz C A, Anderson R E. J Neurochem. 1991;57:1405–1412. doi: 10.1111/j.1471-4159.1991.tb08307.x. [DOI] [PubMed] [Google Scholar]
- 9.Kelleher D J, Johnson G L. J Biol Chem. 1986;261:4749–4757. [PubMed] [Google Scholar]
- 10.Kapoor C L, O’Brien P J, Chader G J. Exp Eye Res. 1987;45:545–556. doi: 10.1016/s0014-4835(87)80065-9. [DOI] [PubMed] [Google Scholar]
- 11.Binder B M, Brewer E, Bownds M D. J Biol Chem. 1989;264:8857–8864. [PubMed] [Google Scholar]
- 12.Wolbring G, Cook N J. Eur J Biochem. 1991;201:601–606. doi: 10.1111/j.1432-1033.1991.tb16320.x. [DOI] [PubMed] [Google Scholar]
- 13.Newton A C, Williams D S. J Biol Chem. 1991;266:17725–17728. [PubMed] [Google Scholar]
- 14.Newton A C, Williams D S. J Biol Chem. 1993;268:18181–18186. [PubMed] [Google Scholar]
- 15.Greene N M, Williams D S, Newton A C. J Biol Chem. 1995;270:6710–6717. doi: 10.1074/jbc.270.12.6710. [DOI] [PubMed] [Google Scholar]
- 16.Zick Y, Sagi-Eisenberg R, Pines M, Gierschik P, Spiegel A M. Proc Natl Acad Sci USA. 1986;83:9294–9297. doi: 10.1073/pnas.83.24.9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayashi F, Lin G Y, Matsumoto H, Yamazaki A. Proc Natl Acad Sci USA. 1991;88:4333–4337. doi: 10.1073/pnas.88.10.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Udovichenko I P, Cunnick J, Gonzalez K, Takemoto D J. Biochem J. 1993;295:49–55. doi: 10.1042/bj2950049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Udovichenko I P, Cunnick J, Gonzalez K, Takemoto D J. J Biol Chem. 1994;269:9850–9856. [PubMed] [Google Scholar]
- 20.Wolbring G, Schnetkamp P P M. Biochemistry. 1995;34:4689–4695. doi: 10.1021/bi00014a024. [DOI] [PubMed] [Google Scholar]
- 21.Weyand I, Kuhn H. Eur J Biochem. 1990;193:459–467. doi: 10.1111/j.1432-1033.1990.tb19360.x. [DOI] [PubMed] [Google Scholar]
- 22.Rhee S G, Choi K D. J Biol Chem. 1992;267:12393–12396. [PubMed] [Google Scholar]
- 23.Sternweis P C, Smrcka A V. Trends Biochem Sci. 1992;17:502–506. doi: 10.1016/0968-0004(92)90340-f. [DOI] [PubMed] [Google Scholar]
- 24.Exton J H. Annu Rev Physiol. 1994;56:349–369. doi: 10.1146/annurev.ph.56.030194.002025. [DOI] [PubMed] [Google Scholar]
- 25.Peng Y-W, Rhee S G, Schoen T, Chader G J, Yau K-W. Soc Neurosci Abstr. 1994;20:965. (abstr.). [Google Scholar]
- 26.Lee C-W, Park D J, Lee K-H, Kim C G, Rhee S G. J Biol Chem. 1993;268:21318–21327. [PubMed] [Google Scholar]
- 27.Lee C-W, Lee K-H, Lee S B, Park D, Rhee S G. J Biol Chem. 1994;269:25335–25338. [PubMed] [Google Scholar]
- 28.Jhon D-Y, Lee H-H, Park D, Lee C-W, Lee K-H, Yoo O J, Rhee S G. J Biol Chem. 1993;268:6654–6661. [PubMed] [Google Scholar]
- 29.Ryu S H, Cho K S, Lee K-Y, Suh P-G, Rhee S G. J Biol Chem. 1987;262:12511–12518. [PubMed] [Google Scholar]
- 30.Taylor S J, Exton J H. FEBS Lett. 1991;286:214–216. doi: 10.1016/0014-5793(91)80976-a. [DOI] [PubMed] [Google Scholar]
- 31.Berstein G, Blank J L, Smrcka A V, Higashijima T, Sternweis P C, Exton J H, Ross E M. J Biol Chem. 1992;267:8081–8088. [PubMed] [Google Scholar]
- 32.Molday R S, Molday L L, Dose A, Clark-Lewis I, Illing M, Cook N J, Eismann E, Kaupp U B. J Biol Chem. 1991;266:21917–21922. [PubMed] [Google Scholar]
- 33.Peng Y-W, Sharp A H, Snyder S H, Yau K-W. Neuron. 1991;6:525–531. doi: 10.1016/0896-6273(91)90055-5. [DOI] [PubMed] [Google Scholar]
- 34.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 35.Ting T D, Goldin S B, Ho Y-K. Methods Neurosci. 1993;15:180–195. [Google Scholar]
- 36.Jiang H, Wu D, Simon M I. J Biol Chem. 1994;269:7593–7596. [PubMed] [Google Scholar]
- 37.Lee C-W, Lee K-H, Lee S B, Park D, Rhee S G. J Biol Chem. 1994;269:25335–25338. [PubMed] [Google Scholar]
- 38.Yau K-W, Baylor D A. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]
- 39.Gerfen C R, Choi W C, Suh P G, Rhee S G. Proc Natl Acad Sci USA. 1988;85:3208–3212. doi: 10.1073/pnas.85.9.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ross C A, MacCumber M W, Glatt C E, Snyder S H. Proc Natl Acad Sci USA. 1989;86:2923–2927. doi: 10.1073/pnas.86.8.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raymond P A, Barthel L K, Rounsifer M E. Exp Neurol. 1992;115:73–78. doi: 10.1016/0014-4886(92)90225-f. [DOI] [PubMed] [Google Scholar]
- 42.Choi W C, Gerfen C R, Suh P G, Rhee S G. Brain Res. 1989;499:193–197. doi: 10.1016/0006-8993(89)91153-0. [DOI] [PubMed] [Google Scholar]
- 43.Mizuguchi M, Yamada M, Kim S U, Rhee S G. Brain Res. 1991;548:35–40. doi: 10.1016/0006-8993(91)91103-8. [DOI] [PubMed] [Google Scholar]
- 44.Simon M I, Strathmann M P, Gautam N. Science. 1991;252:802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- 45.Milligan G, Mullaney I, McCallum F. Biochim Biophys Acta. 1993;1179:208–212. doi: 10.1016/0167-4889(93)90143-d. [DOI] [PubMed] [Google Scholar]
- 46.Milligan G. J Neurochem. 1993;61:845–851. doi: 10.1111/j.1471-4159.1993.tb03595.x. [DOI] [PubMed] [Google Scholar]
- 47.Shapira H, Way J, Lipinsky D, Oron Y, Battey J F. FEBS Lett. 1994;348:89–92. doi: 10.1016/0014-5793(94)00570-2. [DOI] [PubMed] [Google Scholar]
- 48.Day N S, Koutz C A, Anderson R E. Curr Eye Res. 1993;12:981–992. doi: 10.3109/02713689309029224. [DOI] [PubMed] [Google Scholar]
- 49.Ferreira P A, Shortridge R D, Pak W L. Proc Natl Acad Sci USA. 1993;90:6042–6046. doi: 10.1073/pnas.90.13.6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee C-W, Park D J, Lee K-H, Kim C G, Rhee S G. J Biol Chem. 1992;268:21318–21327. [PubMed] [Google Scholar]
- 51.Bloomquist B T, Shortridge R D, Schneuwly S, Perdew M, Montel C, Steller H, Rubin G, Pak W L. Cell. 1988;54:723–733. doi: 10.1016/s0092-8674(88)80017-5. [DOI] [PubMed] [Google Scholar]
- 52.Pak W L, Grossfield J, Arnold K. Nature (London) 1970;227:518–520. doi: 10.1038/227518b0. [DOI] [PubMed] [Google Scholar]
- 53.Hotta Y, Benzer S. Proc Natl Acad Sci USA. 1970;67:1156–1163. doi: 10.1073/pnas.67.3.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferreira P A, Pak W L. J Biol Chem. 1994;269:3129–3131. [PubMed] [Google Scholar]
- 55.Peng Y-W, Robishaw J D, Levine M A, Yau K-W. Proc Natl Acad Sci USA. 1992;89:10882–10886. doi: 10.1073/pnas.89.22.10882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee R H, Lieberman B S, Yamane H K, Bok D, Fung B K-K. J Biol Chem. 1992;267:24776–24781. [PubMed] [Google Scholar]
- 57.Ong O C, Yamane H K, Phan K B, Fong H K W, Bok D, Lee R H, Fung B K-K. J Biol Chem. 1995;270:8495–8500. doi: 10.1074/jbc.270.15.8495. [DOI] [PubMed] [Google Scholar]
- 58.Amatruda T T, Steele D A, Zlepak V Z, Simon M I. Proc Natl Acad Sci USA. 1991;88:5587–5591. doi: 10.1073/pnas.88.13.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilkie T M, Scherle P A, Strathmann M P, Slepak V Z, Simon M L. Proc Natl Acad Sci USA. 1991;88:10049–10053. doi: 10.1073/pnas.88.22.10049. [DOI] [PMC free article] [PubMed] [Google Scholar]