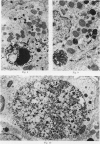
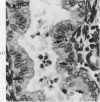
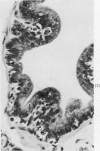
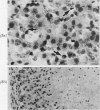
Abstract
The term apoptosis is proposed for a hitherto little recognized mechanism of controlled cell deletion, which appears to play a complementary but opposite role to mitosis in the regulation of animal cell populations. Its morphological features suggest that it is an active, inherently programmed phenomenon, and it has been shown that it can be initiated or inhibited by a variety of environmental stimuli, both physiological and pathological.
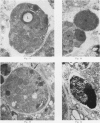
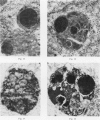
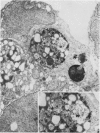
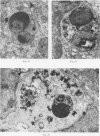
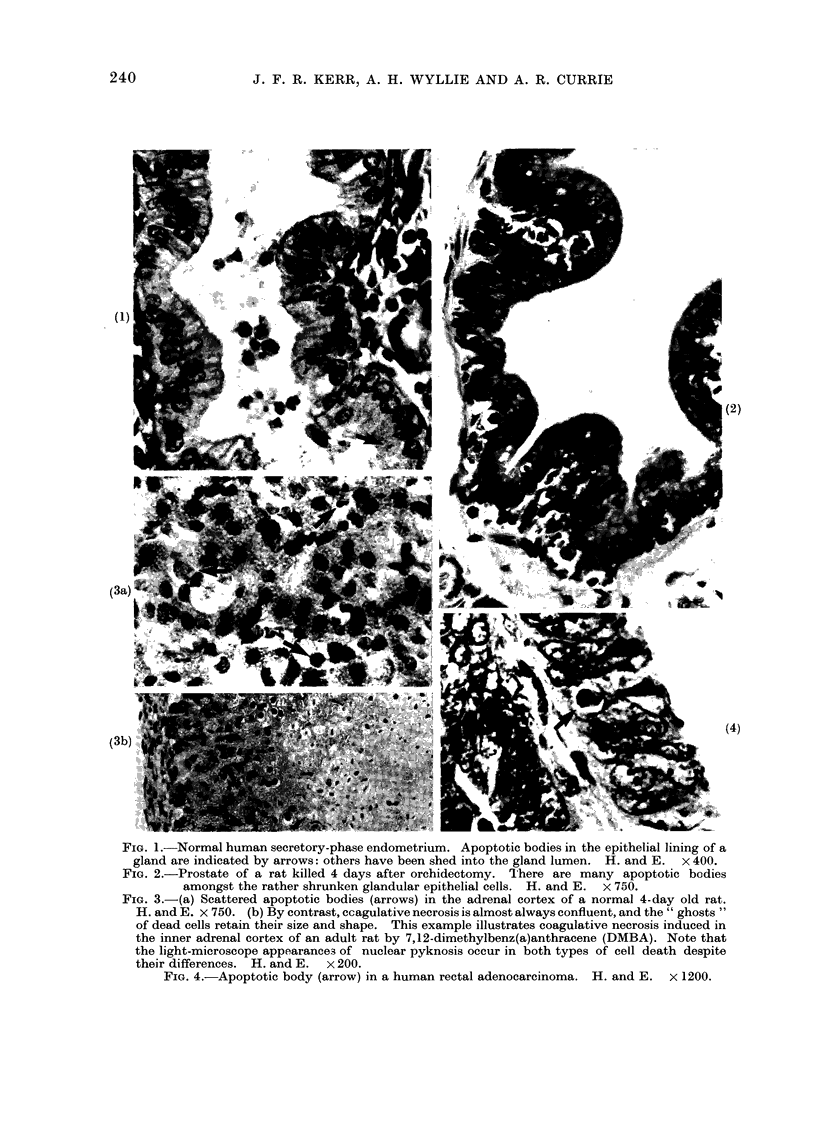
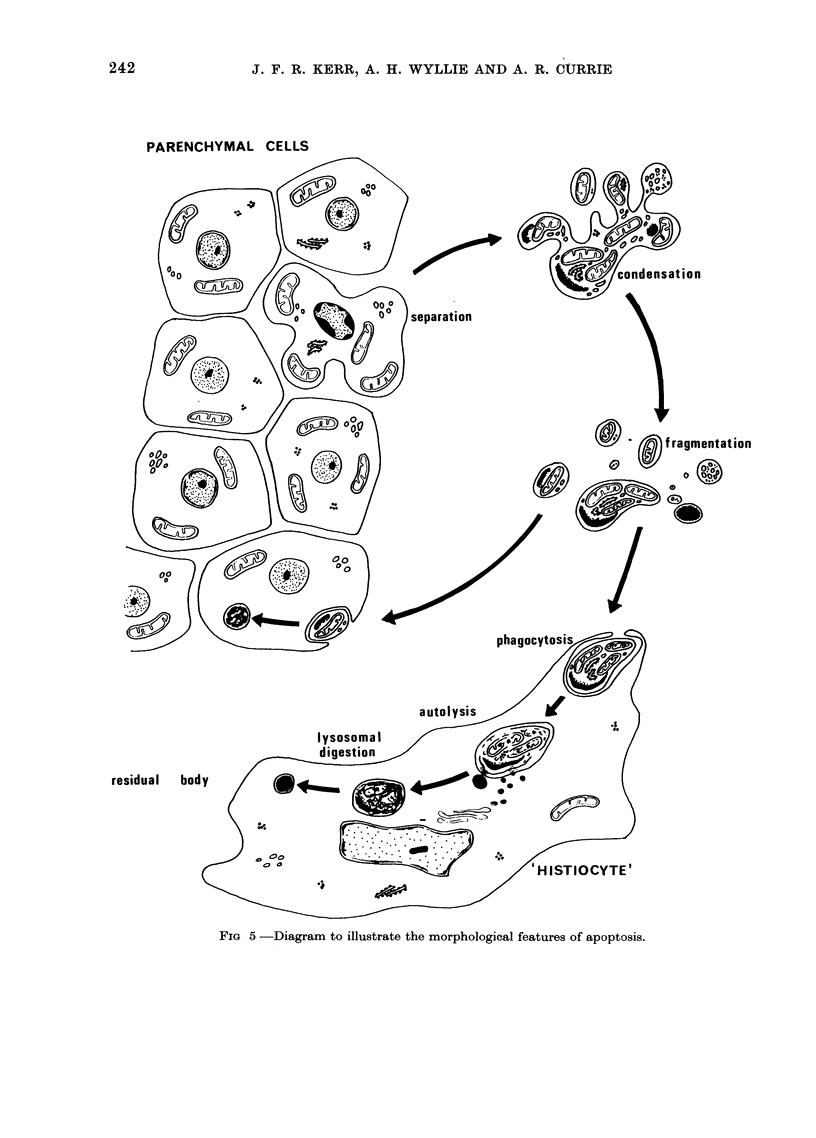
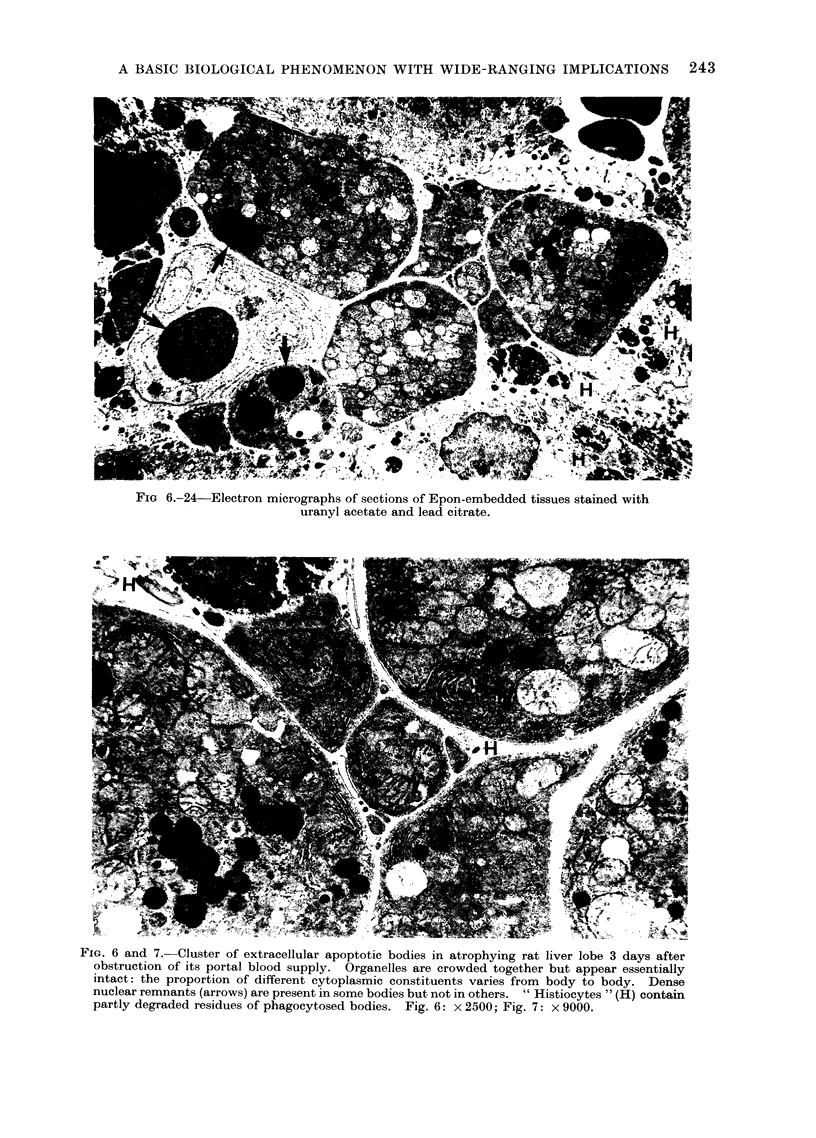
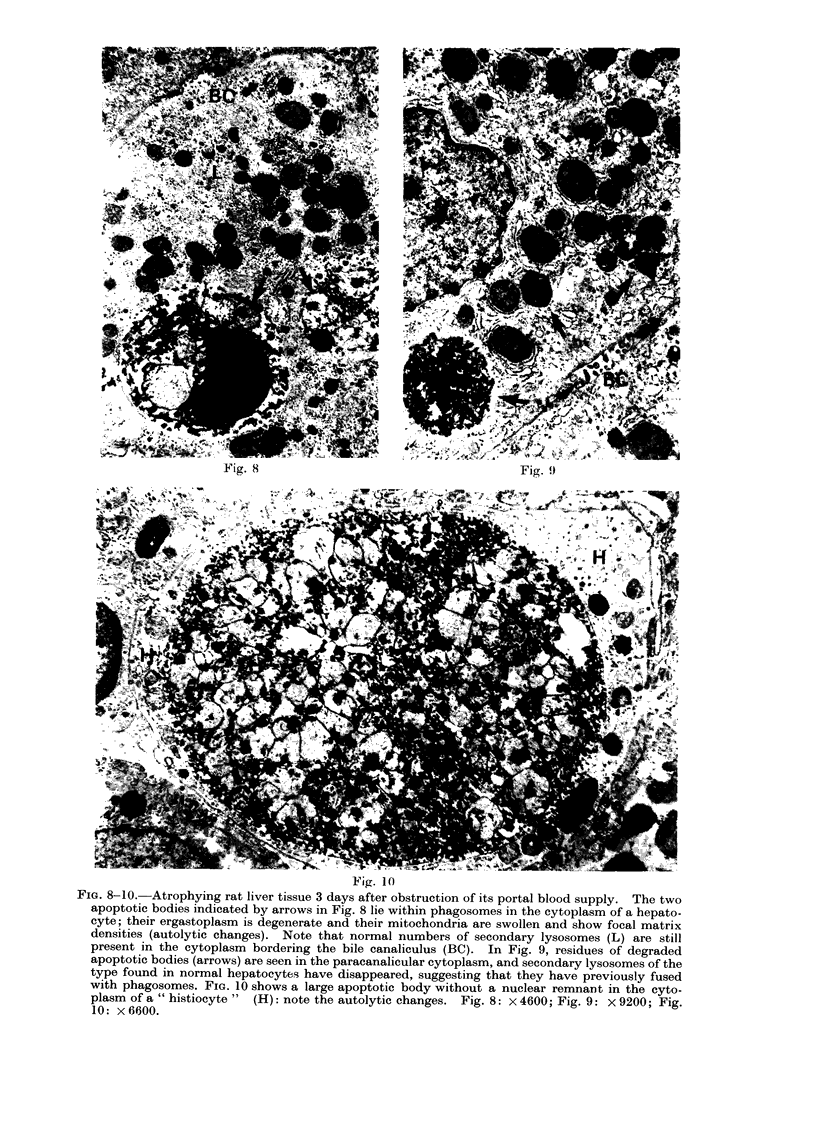
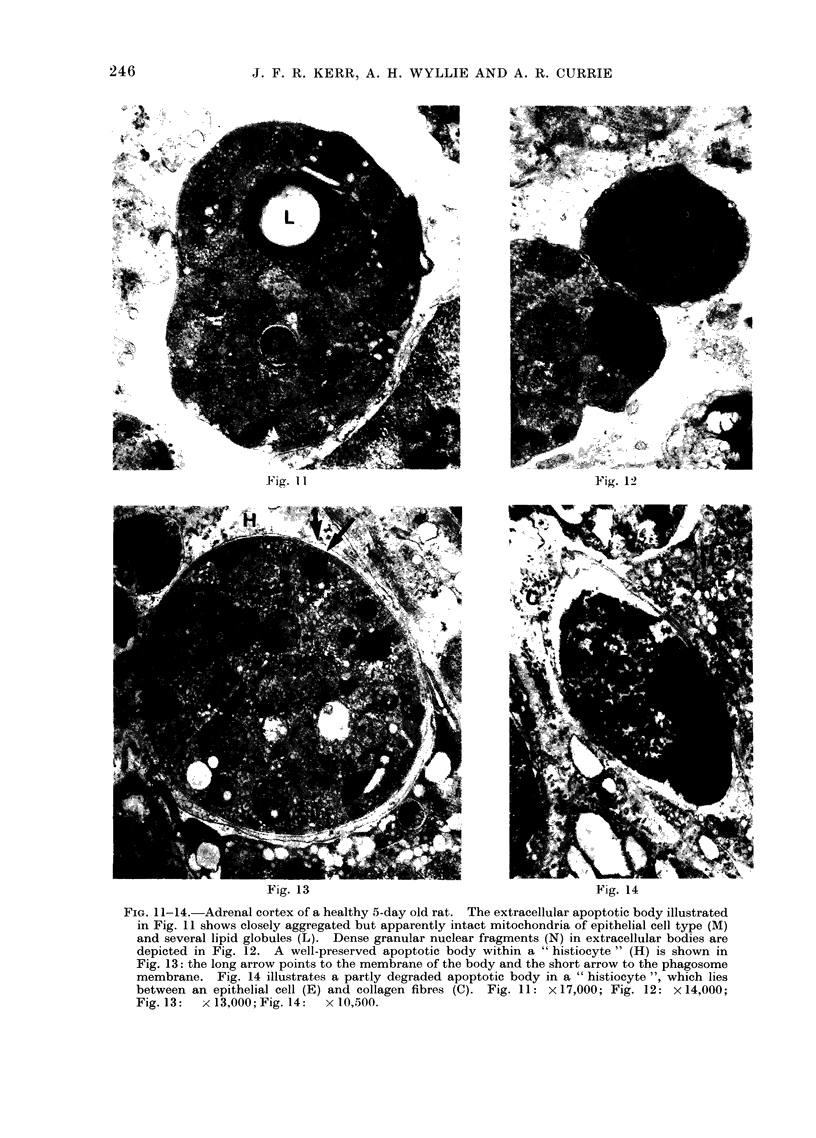
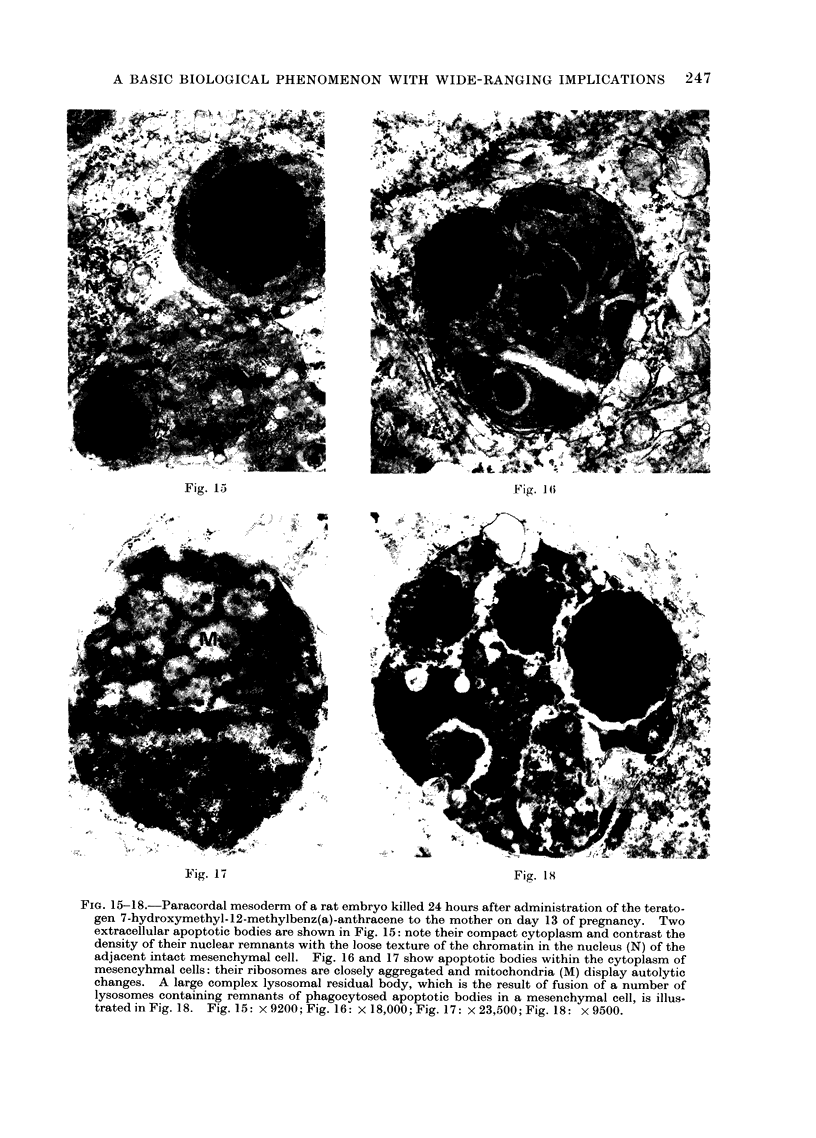
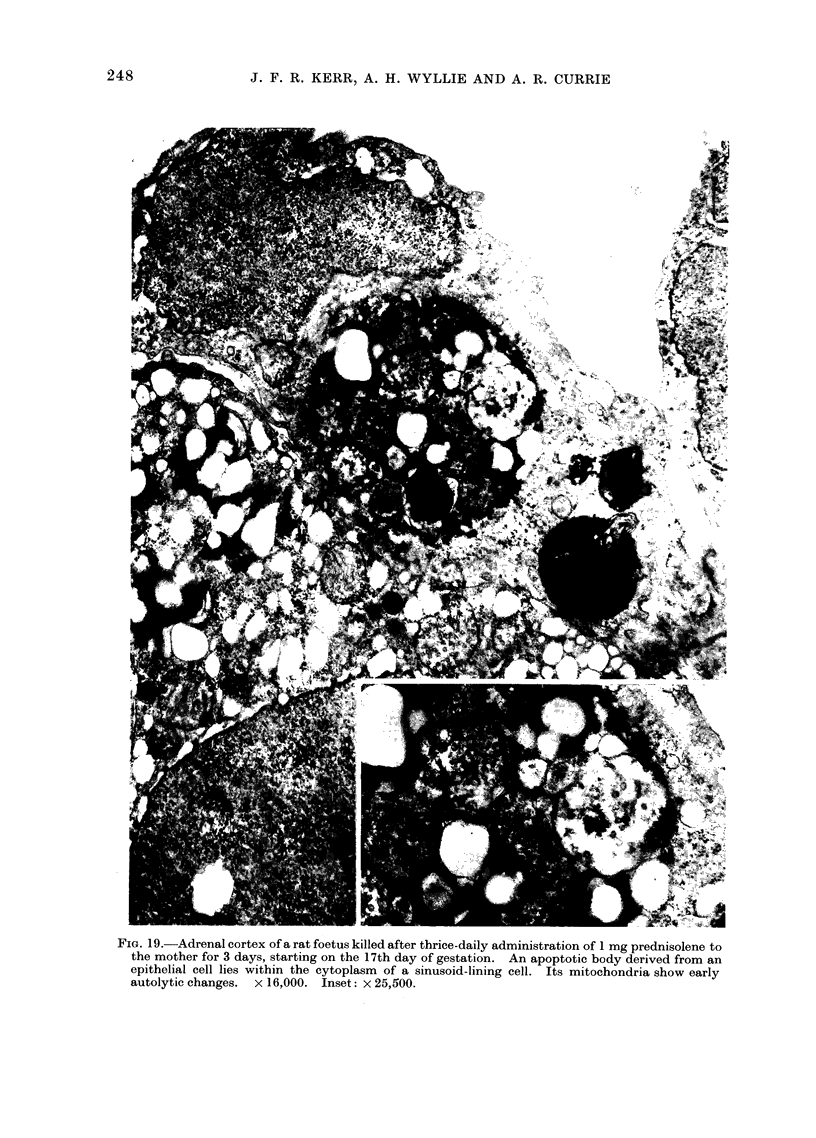
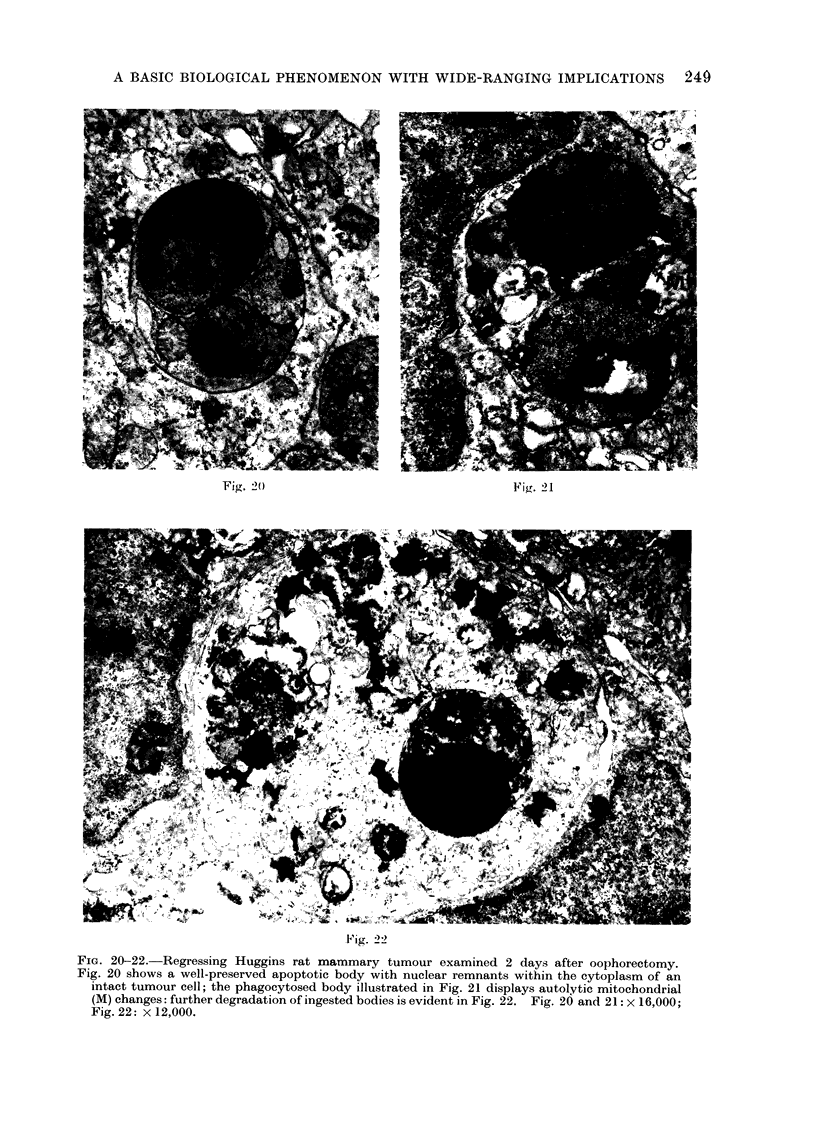
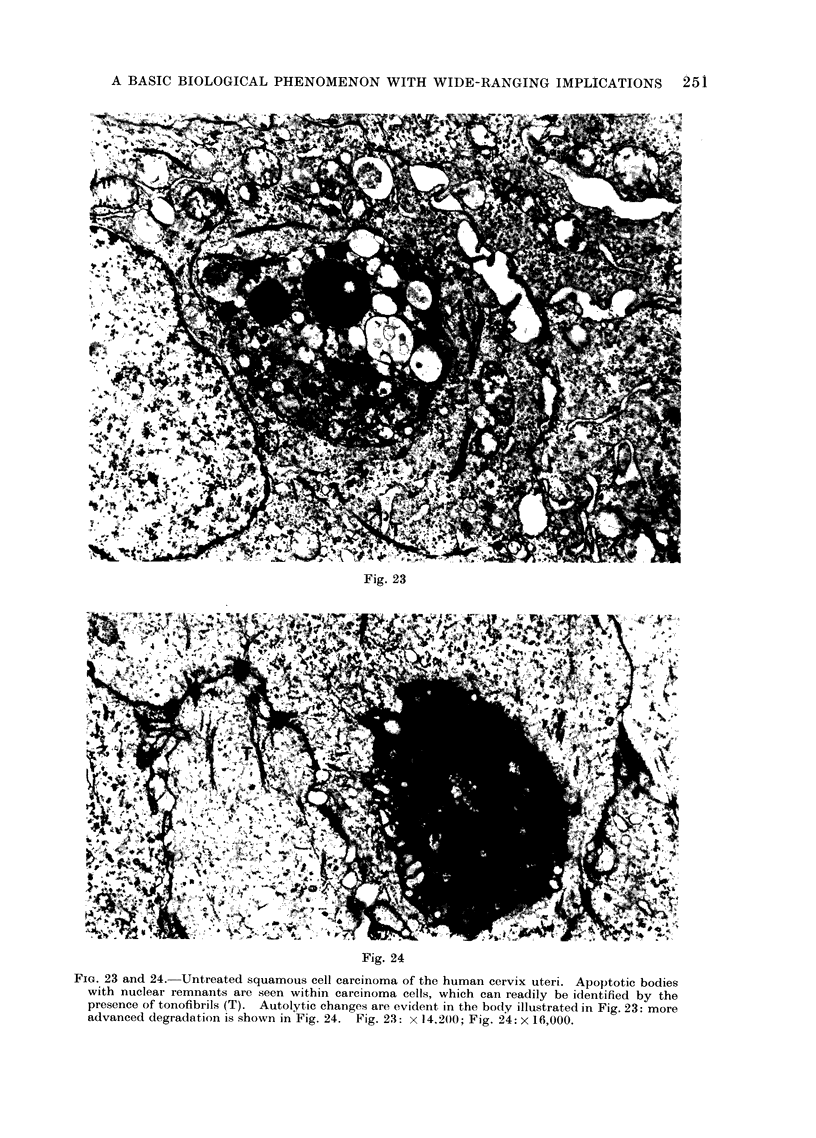
The structural changes take place in two discrete stages. The first comprises nuclear and cytoplasmic condensation and breaking up of the cell into a number of membrane-bound, ultrastructurally well-preserved fragments. In the second stage these apoptotic bodies are shed from epithelial-lined surfaces or are taken up by other cells, where they undergo a series of changes resembling in vitro autolysis within phagosomes, and are rapidly degraded by lysosomal enzymes derived from the ingesting cells.
Apoptosis seems to be involved in cell turnover in many healthy adult tissues and is responsible for focal elimination of cells during normal embryonic development. It occurs spontaneously in untreated malignant neoplasms, and participates in at least some types of therapeutically induced tumour regression. It is implicated in both physiological involution and atrophy of various tissues and organs. It can also be triggered by noxious agents, both in the embryo and adult animal.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham R., Morris M., Hendy R. Lysosomal changes in epithelial cells of the mouse thymus after hydrocortisone treatment. Histochemie. 1969;17(4):295–311. doi: 10.1007/BF00305454. [DOI] [PubMed] [Google Scholar]
- Anton E., Brandes D. Lysosomes in mice mammary tumors treated with cyclophosphamide. Distribution related to course of disease. Cancer. 1968 Mar;21(3):483–500. doi: 10.1002/1097-0142(196803)21:3<483::aid-cncr2820210320>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Ballard K. J., Holt S. J. Cytological and cytochemical studies on cell death and digestion in the foetal rat foot: the role of macrophages and hydrolytic enzymes. J Cell Sci. 1968 Jun;3(2):245–262. doi: 10.1242/jcs.3.2.245. [DOI] [PubMed] [Google Scholar]
- Biava C., Mukhlova-Montiel M. Electron Microscopic Observations on Councilman-Like Acidophilic Bodies and Other Forms of Acidophilic Changes in Human Liver Cells. Am J Pathol. 1965 May;46(5):775–802. [PMC free article] [PubMed] [Google Scholar]
- Clifton K. H., Yatvin M. B. Cell population growth and cell loss in the MTG-B mouse mammary carcinoma. Cancer Res. 1970 Mar;30(3):658–664. [PubMed] [Google Scholar]
- Cole S., Matter A., Karnovsky M. J. Autophagic vacuoles in experimental atrophy. Exp Mol Pathol. 1971 Apr;14(2):158–175. doi: 10.1016/0014-4800(71)90062-1. [DOI] [PubMed] [Google Scholar]
- Frindel E., Malaise E., Tubiana M. Cell proliferation kinetics in five human solid tumors. Cancer. 1968 Sep;22(3):611–620. doi: 10.1002/1097-0142(196809)22:3<611::aid-cncr2820220317>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Helminen H. J., Ericsson J. L., Niemi M. Lysosomal changes during castration-induced prostatic involution in the rat. Acta Pathol Microbiol Scand A. 1970;78(4):493–494. [PubMed] [Google Scholar]
- Helminen H. J., Ericsson J. L. Ultrastructural studies on prostatic involution in the rat. Mechanism of autophagy in epithelial cells, with special reference to the rough-surfaced endoplasmic reticulum. J Ultrastruct Res. 1971 Sep;36(5):708–724. doi: 10.1016/s0022-5320(71)90025-6. [DOI] [PubMed] [Google Scholar]
- Iversen O. H. Kinetics of cellular proliferation and cell loss in human carcinomas. A discussion of methods available for in vivo studies. Eur J Cancer. 1967 Nov;3(4):389–394. doi: 10.1016/0014-2964(67)90023-0. [DOI] [PubMed] [Google Scholar]
- Judah J. D., Ahmed K., McLean A. E. Pathogenesis of cell necrosis. Fed Proc. 1965 Sep-Oct;24(5):1217–1221. [PubMed] [Google Scholar]
- Kerr J. F. A histochemical study of hypertrophy and ischaemic injury of rat liver with special reference to changes in lysosomes. J Pathol Bacteriol. 1965 Oct;90(2):419–435. doi: 10.1002/path.1700900210. [DOI] [PubMed] [Google Scholar]
- Kerr J. F. An electron microscopic study of liver cell necrosis due to albitocin. Pathology. 1970 Oct;2(4):251–259. doi: 10.3109/00313027009077139. [DOI] [PubMed] [Google Scholar]
- Kerr J. F. An electron-microscope study of liver cell necrosis due to heliotrine. J Pathol. 1969 Mar;97(3):557–562. doi: 10.1002/path.1710970314. [DOI] [PubMed] [Google Scholar]
- Kerr J. F. Lysosome changes in acute liver injury due to heliotrine. J Pathol Bacteriol. 1967 Jan;93(1):167–174. doi: 10.1002/path.1700930116. [DOI] [PubMed] [Google Scholar]
- Kerr J. F. Shrinkage necrosis: a distinct mode of cellular death. J Pathol. 1971 Sep;105(1):13–20. doi: 10.1002/path.1711050103. [DOI] [PubMed] [Google Scholar]
- Klion F. M., Schaffner F. The ultrastructure of acidophilic "Councilman-like" bodies in the liver. Am J Pathol. 1966 May;48(5):755–767. [PMC free article] [PubMed] [Google Scholar]
- La Pushin R. W., de Harven E. A study of gluco-corticosteroid-induced pyknosis in the thymus and lymph node of the adrenalectomized rat. J Cell Biol. 1971 Sep;50(3):583–597. doi: 10.1083/jcb.50.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lala P. K. Evaluation of the mode of cell death in Ehrlich ascites tumor. Cancer. 1972 Jan;29(1):261–266. doi: 10.1002/1097-0142(197201)29:1<261::aid-cncr2820290139>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Makman M. H., Nakagawa S., White A. Studies of the mode of action of adrenal steroids on lymphocytes. Recent Prog Horm Res. 1967;23:195–227. doi: 10.1016/b978-1-4831-9826-2.50008-8. [DOI] [PubMed] [Google Scholar]
- Moppert J., von Ekesparre D., Bianchi L. Zur Morphogenese der eosinophilen Einzelzellnekrose im Leberparenchym des Menschen. Eine licht- und elektronenoptisch korrelierte Untersuchung. Virchows Arch Pathol Anat Physiol Klin Med. 1967 Jun 12;342(3):210–220. [PubMed] [Google Scholar]
- Paris J. E., Brandes D. Effect of x-irradiation on the functional status of lysosomal enzymes of mouse mammary gland carcinomas. Cancer Res. 1971 Apr;31(4):392–401. [PubMed] [Google Scholar]
- Refsum S. B., Berdal P. Cell loss in malignant tumours in man. Eur J Cancer. 1967 Aug;3(3):235–236. doi: 10.1016/0014-2964(67)90049-7. [DOI] [PubMed] [Google Scholar]
- SWARTZENDRUBER D. C., CONGDON C. C. ELECTRON MICROSCOPE OBSERVATIONS ON TINGIBLE BODY MACROPHAGES IN MOUSE SPLEEN. J Cell Biol. 1963 Dec;19:641–646. doi: 10.1083/jcb.19.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J. W., Jr Death in embryonic systems. Science. 1966 Nov 4;154(3749):604–612. doi: 10.1126/science.154.3749.604. [DOI] [PubMed] [Google Scholar]
- Steel G. G. Cell loss as a factor in the growth rate of human tumours. Eur J Cancer. 1967 Nov;3(4):381–387. doi: 10.1016/0014-2964(67)90022-9. [DOI] [PubMed] [Google Scholar]
- TRUMP B. F., GOLDBLATT P. J., STOWELL R. E. STUDIES ON NECROSIS OF MOUSE LIVER IN VITRO. ULTRASTRUCTURAL ALTERATIONS IN THE MITOCHONDRIA OF HEPATIC PARENCHYMAL CELLS. Lab Invest. 1965 Apr;14:343–371. [PubMed] [Google Scholar]
- Webster D. A., Gross J. Studies on possible mechanisms of programmed cell death in the chick embryo. Dev Biol. 1970 May;22(1):157–184. doi: 10.1016/0012-1606(70)90012-6. [DOI] [PubMed] [Google Scholar]
- Weinstein G. D., Frost P. Cell proliferation in human basal cell carcinoma. Cancer Res. 1970 Mar;30(3):724–728. [PubMed] [Google Scholar]
- Wilgram G. F., Kidd R. L., Krawczyk W. S., Cole P. L. Sunburn effect on keratinosomes. A report with special note on ultraviolet-induced dyskeratosis. Arch Dermatol. 1970 May;101(5):505–519. doi: 10.1001/archderm.101.5.505. [DOI] [PubMed] [Google Scholar]
- van Haelst U. Light and electron microscopic study of the normal and pathological thymus of the rat. II. The acute thymic involution. Z Zellforsch Mikrosk Anat. 1967;80(2):153–182. doi: 10.1007/BF00337454. [DOI] [PubMed] [Google Scholar]