Abstract
(1) The antigenic activity of basic protein extracts of a variety of human tissues towards sensitized lymphocytes from cancer patients has been studied using the macrophage electrophoretic migration method.
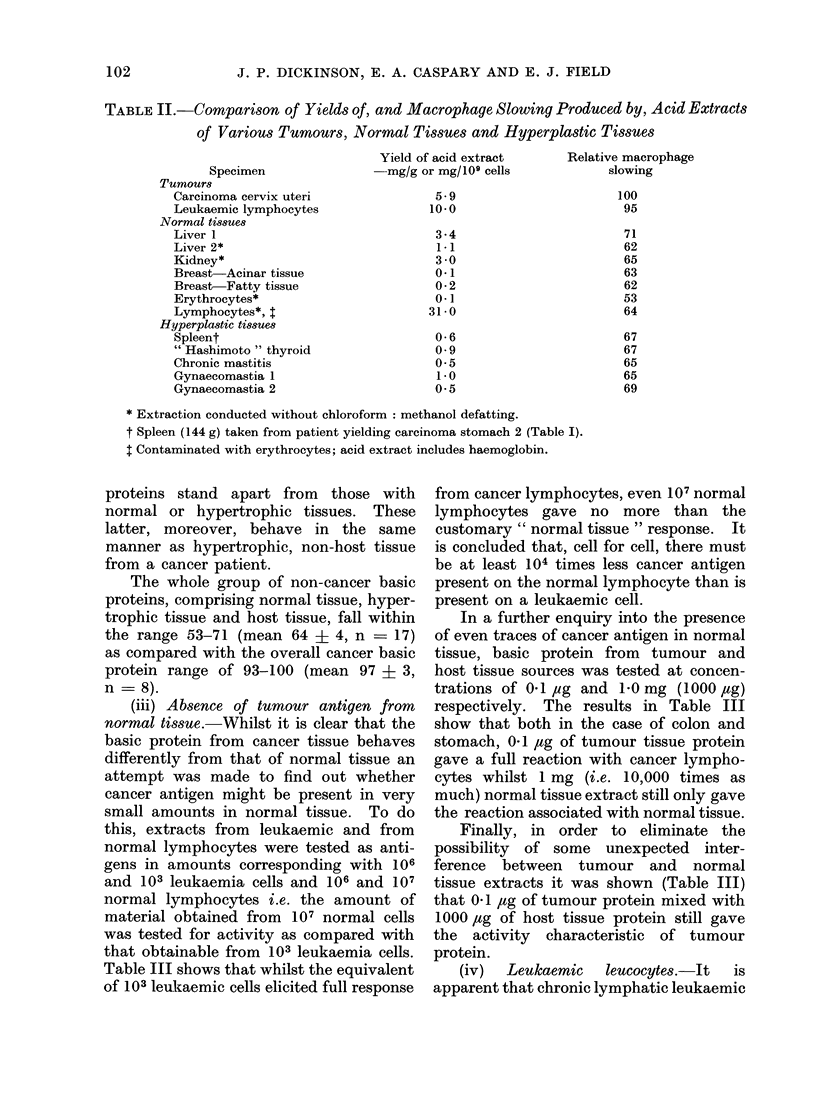
(2) Basic protein prepared from human tumour tissue has antigenic properties which differ from basic protein prepared from normal tissues, from hyperplastic tissue and even tumour bearing host tissue.
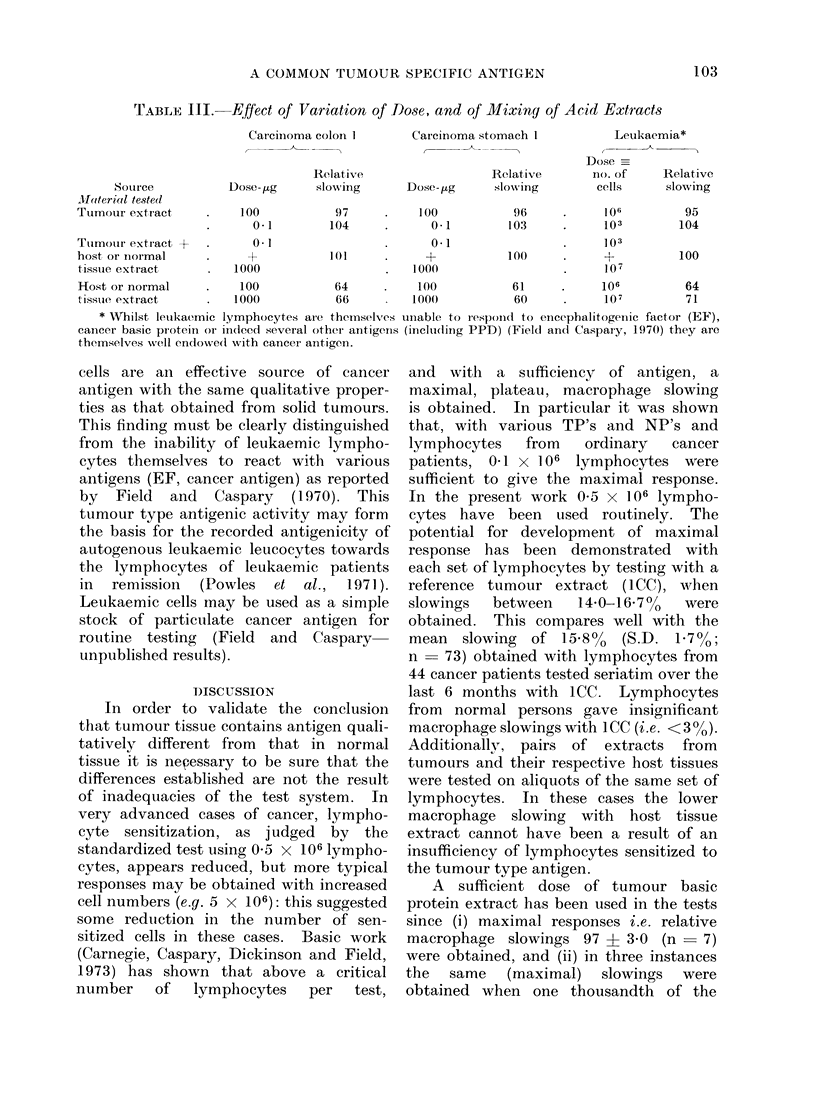
(3) Tumour type antigenic molecules are at least 104 times less numerous on normal cells than on tumour cells. If our reasoning is accepted then they are absent from normal cells, and thus restricted in vivo to malignant cells.
(4) Hyperplastic tissues (gynaecomastia, etc.) do not contain cancer basic protein.
(5) Human chronic lymphatic leukaemic leucocytes possess the same type of cancer antigenic activity as solid tumours. This antigenic activity is not shared by normal lymphocytes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BJORKLUND B., BJORKLUND V. Antigenicity of pooled human malignant and normal tissues by cyto-immunological technique; presence of an insoluble, heat-labile tumor antigen. Int Arch Allergy Appl Immunol. 1957;10(3):153–184. [PubMed] [Google Scholar]
- Baldwin R. W., Glaves D. Solubilization of tumour-specific antigen from plasma membrane of an aminoazo-dye-induced rat hepatoma. Clin Exp Immunol. 1972 May;11(1):51–56. [PMC free article] [PubMed] [Google Scholar]
- Björklund B. Systematic antigenic change in human carcinoma tissues by hemagglutination techniques. Int Arch Allergy Appl Immunol. 1969;36(1):191–203. doi: 10.1159/000230741. [DOI] [PubMed] [Google Scholar]
- Bloom B. R. In vitro methods in cell-mediated immunity in man. N Engl J Med. 1971 May 27;284(21):1212–1213. doi: 10.1056/NEJM197105272842111. [DOI] [PubMed] [Google Scholar]
- Bubeník J., Perlmann P., Helmstein K., Moberger G. Immune response to urinary bladder tumours in man. Int J Cancer. 1970 Jan 15;5(1):39–46. doi: 10.1002/ijc.2910050106. [DOI] [PubMed] [Google Scholar]
- COULSON A. S., CHALMERS D. G. SEPARATION OF VIABLE LYMPHOCYTES FROM HUMAN BLOOD. Lancet. 1964 Feb 29;1(7331):468–469. doi: 10.1016/s0140-6736(64)90799-8. [DOI] [PubMed] [Google Scholar]
- Carnegie P. R., Caspary E. A., Field E. J. Identification of a tumour antigen. Biochem J. 1972 Feb;126(3):5P–6P. doi: 10.1042/bj1260005p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary E. A., Field E. J. Specific lymphocyte sensitization in cancer: is there a common antigen in human malignant neoplasia? Br Med J. 1971 Jun 12;2(5762):613–617. doi: 10.1136/bmj.2.5762.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson J. P., Caspary E. A., Field E. J. Localization of tumour specific antigen on external surface of plasma membrane. Nat New Biol. 1972 Oct 11;239(93):181–183. doi: 10.1038/newbio239181a0. [DOI] [PubMed] [Google Scholar]
- Field E. J., Caspary E. A. Demonstration of sensitized lymphocytes in blood. J Clin Pathol. 1971 Mar;24(2):179–181. doi: 10.1136/jcp.24.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field E. J., Caspary E. A. Lymphocyte sensitisation: an in-vitro test for cancer? Lancet. 1970 Dec 26;2(7687):1337–1341. doi: 10.1016/s0140-6736(70)92361-5. [DOI] [PubMed] [Google Scholar]
- Field E. J., Caspary E. A. Lymphocyte sensitization in advanced malignant disease: a study of serum lymphocyte depressive factor. Br J Cancer. 1972 Jun;26(3):164–173. doi: 10.1038/bjc.1972.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashim G. A., Eylar E. H. Allergic encephalomyelitis: enzymatic degradation of the encephalitogenic basic protein from bovine spinal cord. Arch Biochem Biophys. 1969 Feb;129(2):635–644. doi: 10.1016/0003-9861(69)90224-0. [DOI] [PubMed] [Google Scholar]
- Hughes D., Caspary E. A. Lymphocyte transformation in vitro measured by tritiated thymidine uptake. I. Lymphocyte culture techniques. Int Arch Allergy Appl Immunol. 1970;37(5):506–531. doi: 10.1159/000230240. [DOI] [PubMed] [Google Scholar]
- Powles R. L., Balchin L. A., Fairley G. H., Alexander P. Recognition of leukaemia cells as foreign before and after autoimmunization. Br Med J. 1971 Feb 27;1(5747):486–489. doi: 10.1136/bmj.1.5747.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard J. A., Moore J. L., Sutherland W. H., Joslin C. A. Macrophage-electrophoretic-mobility (M.E.M.) test for malignant disease. An independent confirmation. Lancet. 1972 Sep 23;2(7778):627–629. doi: 10.1016/s0140-6736(72)93018-8. [DOI] [PubMed] [Google Scholar]
- TASHIAN R. E. Multiple forms of esterases from human erythrocytes. Proc Soc Exp Biol Med. 1961 Nov;108:364–366. doi: 10.3181/00379727-108-26939. [DOI] [PubMed] [Google Scholar]
- Taranger L. A., Chapman W. H., Hellström I., Hellström K. E. Immunological studies on urinary bladder tumors of rats and mice. Science. 1972 Jun 23;176(4041):1337–1340. doi: 10.1126/science.176.4041.1337. [DOI] [PubMed] [Google Scholar]


