Abstract
The records of 1243 patients with myasthenia gravis (M.G.) have been reviewed in a retrospective study of the incidence of extrathymic neoplasms. Ninety-four malignant neoplasms were traced.
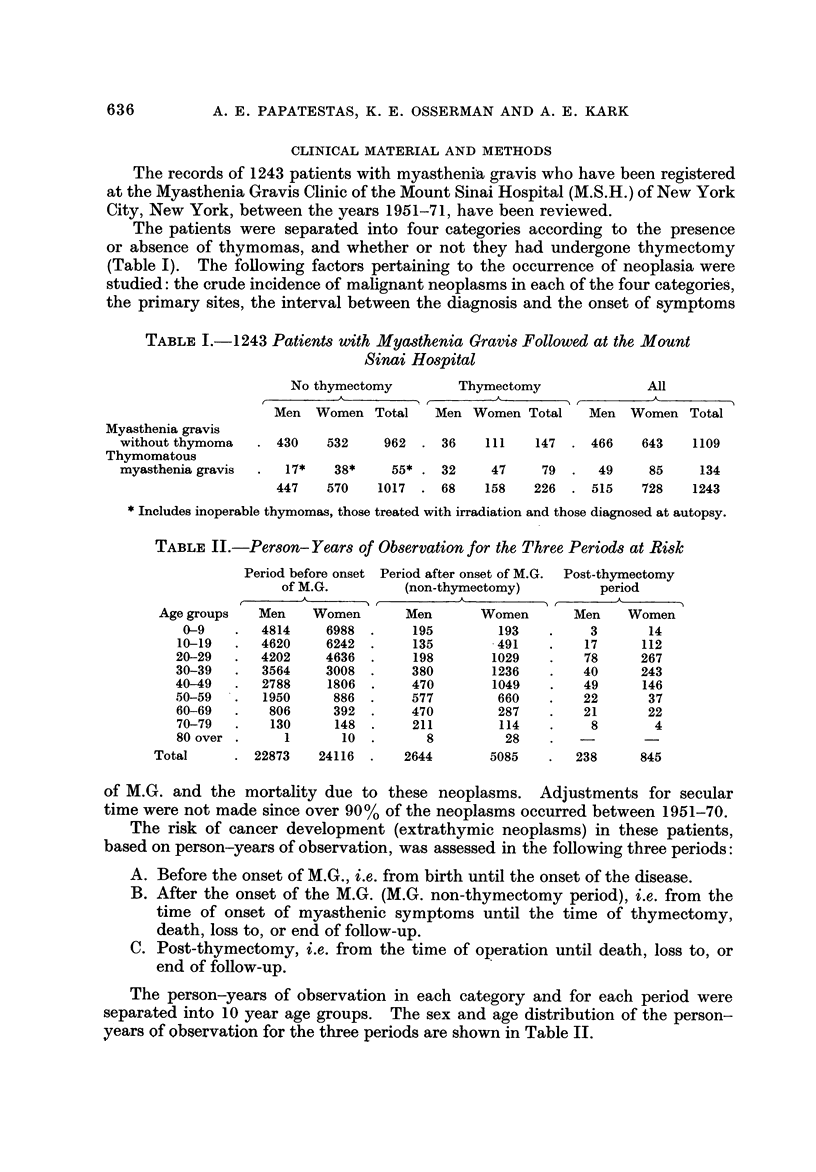
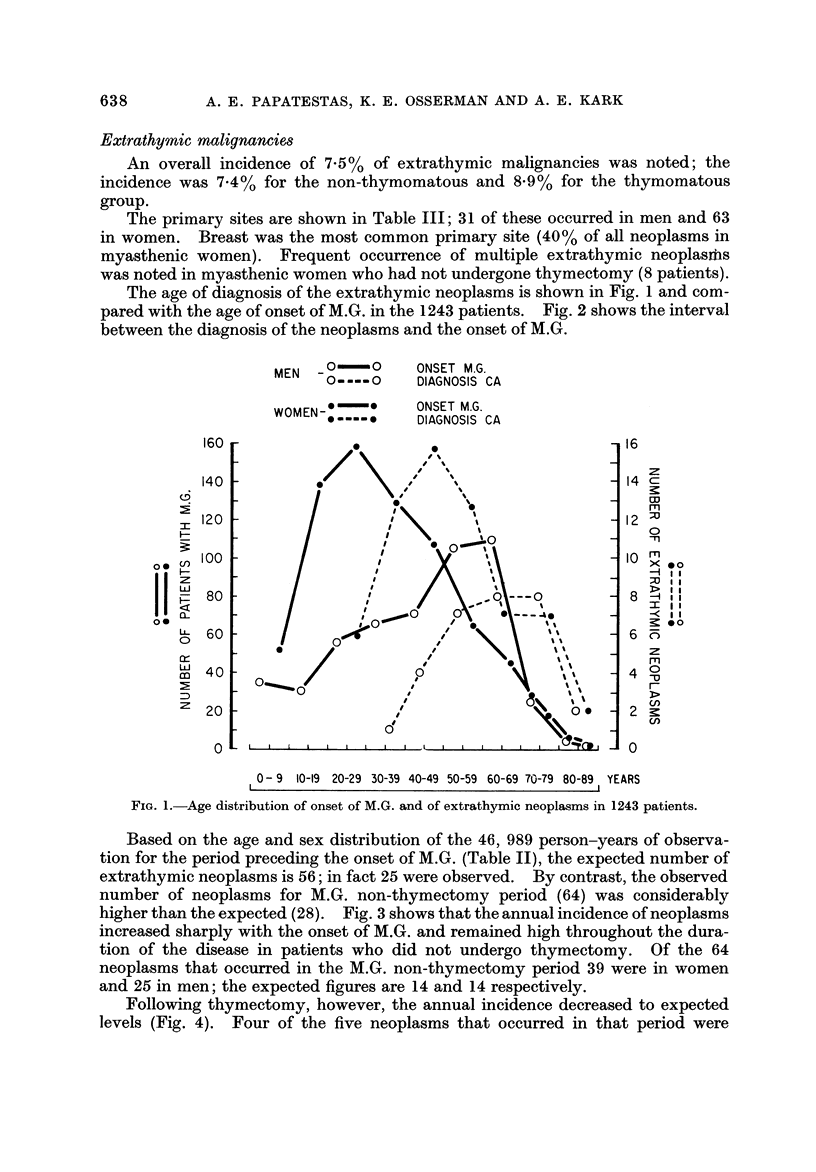
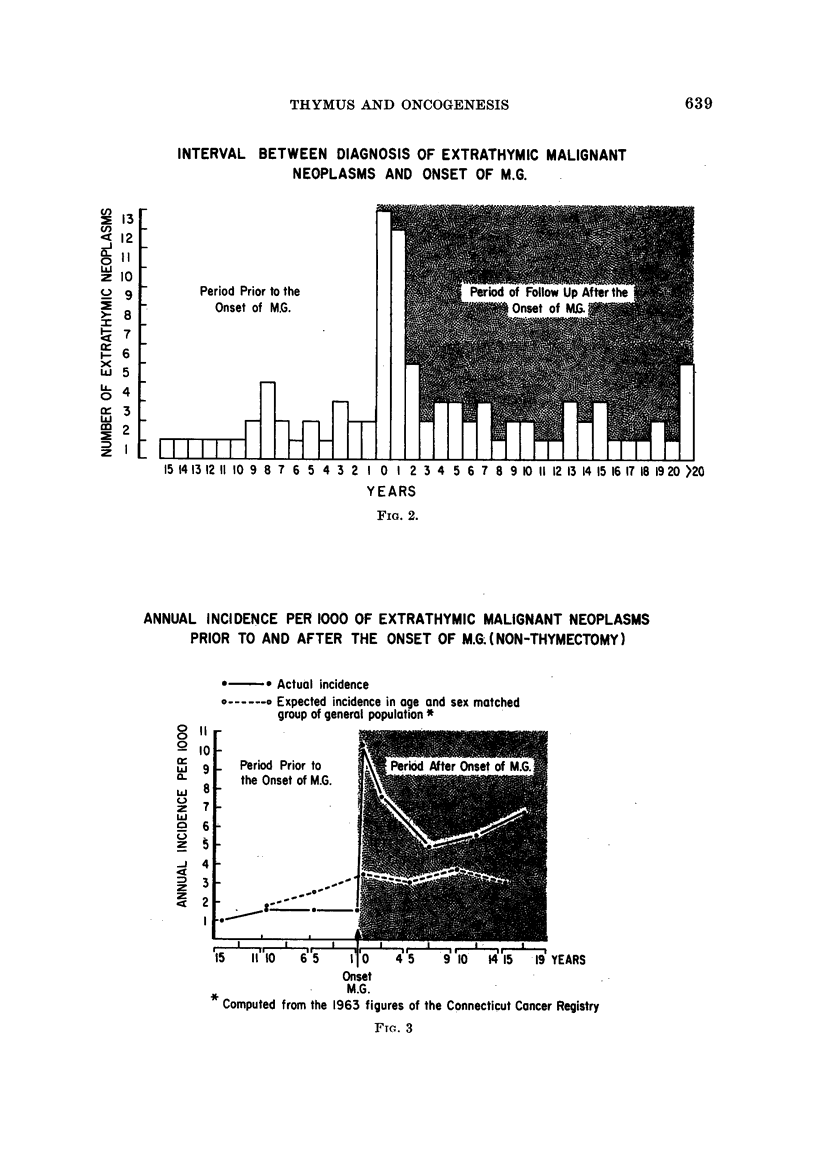
The onset of the disease (M.G.) coincided with a marked increase in the incidence of extrathymic neoplasms. The observed number of neoplasms in the year of onset of M.G. was three times higher than the expected in a control group. This was in sharp contrast to the lower than expected incidence in the years preceding the onset of M.G.
The incidence remained at higher than the expected levels throughout the course of the disease in patients who did not undergo thymectomy, while in those patients who had thymectomy the incidence decreased to the levels of the general population after the second postoperative year.
These observations suggest an oncogenic thymic influence. The possibility is discussed of the potential oncogenic role of abnormal clones of immunocompetent small lymphocytes of thymic origin.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen S. M., Waxman S. Myasthenia gravis, chronic lymphocytic leukemia, and autoimmune hemolytic anemia. "A spectrum of thymic abnormalities? Arch Intern Med. 1967 Dec;120(6):717–720. [PubMed] [Google Scholar]
- Goldenberg G. J., Paraskevas F., Israels L. G. The association of rheumatoid arthritis with plasma cell and lymphocytic neoplasms. Arthritis Rheum. 1969 Dec;12(6):569–579. doi: 10.1002/art.1780120604. [DOI] [PubMed] [Google Scholar]
- Miller D. G. The association of immune disease and malignant lymphoma. Ann Intern Med. 1967 Mar;66(3):507–521. doi: 10.7326/0003-4819-66-3-507. [DOI] [PubMed] [Google Scholar]
- Papatestas A. E., Alpert L. I., Osserman K. E., Osserman R. S., Kark A. E. Studies in myasthenia gravis: effects of thymectomy. Results on 185 patients with nonthymomatous and thymomatous myasthenia gravis, 1941-1969. Am J Med. 1971 Apr;50(4):465–474. doi: 10.1016/0002-9343(71)90336-6. [DOI] [PubMed] [Google Scholar]
- Perlo V. P., Arnason B., Poskanzer D., Castleman B., Schwab R. S., Osserman K. E., Papatestis A., Alpert L., Kark A. The role of thymectomy in the treatment of myasthenia gravis. Ann N Y Acad Sci. 1971 Sep 15;183:308–315. doi: 10.1111/j.1749-6632.1971.tb30761.x. [DOI] [PubMed] [Google Scholar]
- SIMPSON J. A. An evaluation of thymectomy in myasthenia gravis. Brain. 1958 Mar;81(1):112–144. doi: 10.1093/brain/81.1.112. [DOI] [PubMed] [Google Scholar]
- Souadjian J. V., Silverstein M. N., Titus J. L. Thymoma and cancer. Cancer. 1968 Dec;22(6):1221–1225. doi: 10.1002/1097-0142(196811)22:6<1221::aid-cncr2820220619>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Wolf S. M., Rowland L. P., Schotland D. L., McKinney A. S., Hoefer P. F., Aranow H., Jr Myasthenia as an autoimmune disease: clinical aspects. Ann N Y Acad Sci. 1966 Jan 26;135(1):517–535. doi: 10.1111/j.1749-6632.1966.tb45500.x. [DOI] [PubMed] [Google Scholar]
- Yunis E. J., Martinez C., Smith J., Stutman O., Good R. A. Spontaneous mammary adenocarcinoma in mice: influence of thymectomy and reconstitution with thymus grafts or spleen cells. Cancer Res. 1969 Jan;29(1):174–178. [PubMed] [Google Scholar]


