Abstract
The tumour specific transplantation antigen (TSTA) from a chemically induced rat sarcoma has been isolated as an electrophoretically homogeneous soluble material by affinity chromatography using a Sepharose bound antibody raised to the tumour in syngeneic rats. The TSTA is specific for the particular tumour used (the MC-1 sarcoma) and does not cross-react with material extracted from other rat sarcomata. In addition, a material with different physicochemical properties which cross-reacted with different sarcomata was also eluted from the antibody column and this may be the previously identified onco-embryonic antigen (OEA1) which is immunogenic in the syngeneic host.
The purified TSTA labelled with 125I was used in a radioimmunoassay which detected soluble TSTA in rats bearing a MC-1 sarcoma. The assay shows that tumour transplantation is associated with a persisting release of soluble antigen into the circulation. This antigenic burden is present continuously and renewed as long as the tumour mass exists.
Full text
PDF


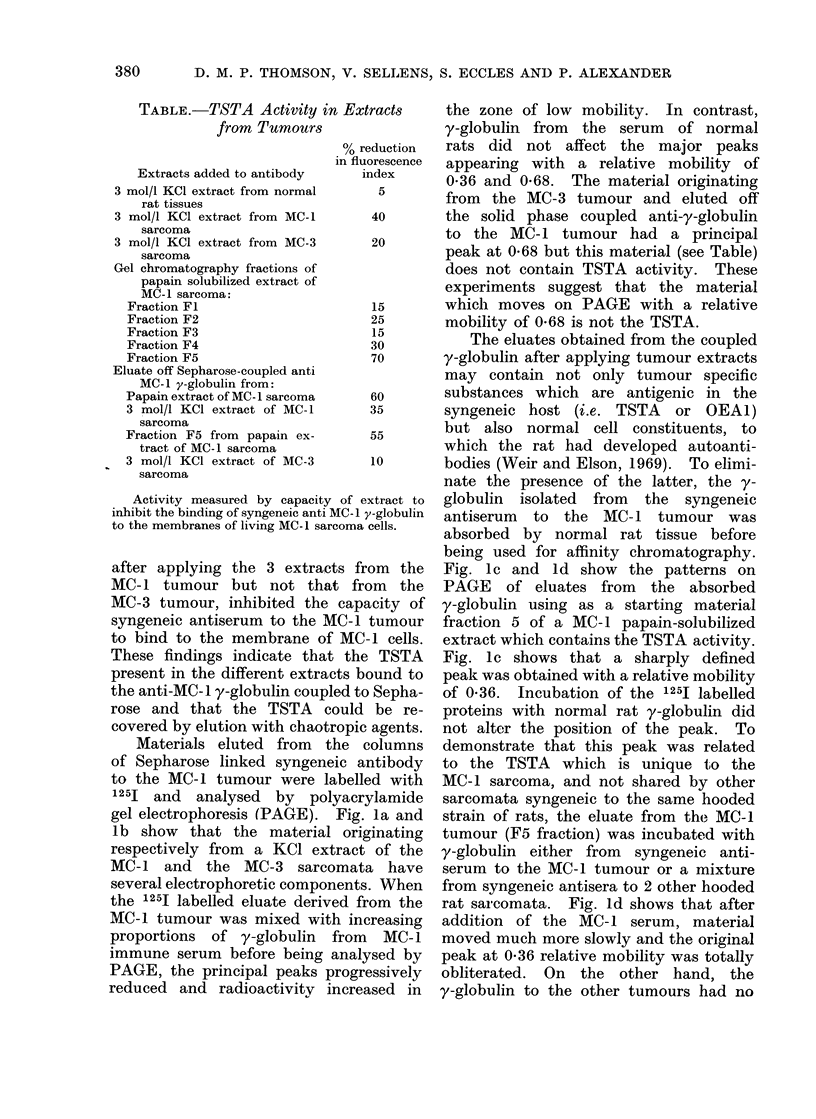





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpers J. H., Steward M. W., Soothill J. F. Differences in immune elimination in inbred mice. The role of low affinity antibody. Clin Exp Immunol. 1972 Sep;12(1):121–132. [PMC free article] [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorbents. Immunochemistry. 1969 Jan;6(1):53–66. doi: 10.1016/0019-2791(69)90178-5. [DOI] [PubMed] [Google Scholar]
- Baldwin R. W., Barker C. R. Demonstration of tumour-specific humoral antibody against aminoazo dye-induced rat hepatomata. Br J Cancer. 1967 Dec;21(4):793–800. doi: 10.1038/bjc.1967.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin R. W., Bowen J. G., Price M. R. Detection of circulating hepatoma D23 antigen and immune complexes in tumour bearer serum. Br J Cancer. 1973 Jul;28(1):16–24. doi: 10.1038/bjc.1973.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin R. W., Embleton M. J., Price M. R. Inhibition of lymphocyte cytotoxicity for human colon carcinoma by treatment with solubilized tumour membrane fractions. Int J Cancer. 1973 Jul 15;12(1):84–92. doi: 10.1002/ijc.2910120109. [DOI] [PubMed] [Google Scholar]
- Baldwin R. W., Embleton M. J., Robins R. A. Cellular and humoral immunity to rat hepatoma-specific antigens correlated with tumour status. Int J Cancer. 1973 Jan 15;11(1):1–10. doi: 10.1002/ijc.2910110102. [DOI] [PubMed] [Google Scholar]
- Baldwin R. W., Glaves D. Solubilization of tumour-specific antigen from plasma membrane of an aminoazo-dye-induced rat hepatoma. Clin Exp Immunol. 1972 May;11(1):51–56. [PMC free article] [PubMed] [Google Scholar]
- Bias W. B., Santos G. W., Burke P. J., Mullins G. M., Humphrey R. L. Cytotoxic antibody in normal human serums reactive with tumor cells from acute lymphocytic leukemia. Science. 1972 Oct 20;178(4058):304–306. doi: 10.1126/science.178.4058.304. [DOI] [PubMed] [Google Scholar]
- Brawn R. J. In vitro desensitization of sensitized murine lymphocytes by a serum factor (soluble antigen?). Proc Natl Acad Sci U S A. 1971 Jul;68(7):1634–1638. doi: 10.1073/pnas.68.7.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Currie G. A. Effect of active immunization with irradiated tumour cells on specific serum inhibitors of cell-mediated immunity in patients with disseminated cancer. Br J Cancer. 1973 Jul;28(1):25–35. doi: 10.1038/bjc.1973.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie G. A., Gage J. O. Influence of tumour growth on the evolution of cytotoxic lymphoid cells in rats bearing a spontaneously metastasizing syngeneic fibrosarcoma. Br J Cancer. 1973 Aug;28(2):136–146. doi: 10.1038/bjc.1973.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström I., Sjögren H. O., Warner G., Hellström K. E. Blocking of cell-mediated tumor immunity by sera from patients with growing neoplasms. Int J Cancer. 1971 Mar 15;7(2):226–237. doi: 10.1002/ijc.2910070206. [DOI] [PubMed] [Google Scholar]
- Holmes E. C., Kahan B. D., Morton D. L. Soluble tumor-specific transplantation antigens from methylcholanthrene-induced guinea pig sarcomas. Cancer. 1970 Feb;25(2):373–379. doi: 10.1002/1097-0142(197002)25:2<373::aid-cncr2820250215>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Lewis M. G., Ikonopisov R. L., Nairn R. C., Phillips T. M., Fairley G. H., Bodenham D. C., Alexander P. Tumour-specific antibodies in human malignant melanoma and their relationship to the extent of the disease. Br Med J. 1969 Sep 6;3(5670):547–552. doi: 10.1136/bmj.3.5670.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer M. S., Leonard E. J., Rapp H. J., Borsos T. Tumor-specific antigen solubilized by hypertonic potassium chloride. J Natl Cancer Inst. 1971 Sep;47(3):703–709. [PubMed] [Google Scholar]
- OLD L. J., BOYSE E. A. IMMUNOLOGY OF EXPERIMENTAL TUMORS. Annu Rev Med. 1964;15:167–186. doi: 10.1146/annurev.me.15.020164.001123. [DOI] [PubMed] [Google Scholar]
- Prehn R. T. The immune reaction as a stimulator of tumor growth. Science. 1972 Apr 14;176(4031):170–171. doi: 10.1126/science.176.4031.170. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Seppälä M. -Foetoprotein in normal human serum. Nature. 1972 Jan 21;235(5334):161–162. doi: 10.1038/235161a0. [DOI] [PubMed] [Google Scholar]
- Sanderson A. R., Cresswell P., Welsh K. I. Involvement of carbohydrate in the immunochemical determinant area of HL-A substances. Nat New Biol. 1971 Mar 3;230(1):8–12. doi: 10.1038/newbio230008a0. [DOI] [PubMed] [Google Scholar]
- Thomson D. M., Alexander P. A cross-reacting embryonic antigen in the membrane of rat sarcoma cells which is immunogenic in the syngeneic host. Br J Cancer. 1973 Jan;27(1):35–47. doi: 10.1038/bjc.1973.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson D. M., Eccles S., Alexander P. Antibodies and soluble tumour-specific antigens in blood and lymph of rats with chemically induced sarcomata. Br J Cancer. 1973 Jul;28(1):6–15. doi: 10.1038/bjc.1973.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson D. M., Krupey J., Freedman S. O., Gold P. The radioimmunoassay of circulating carcinoembryonic antigen of the human digestive system. Proc Natl Acad Sci U S A. 1969 Sep;64(1):161–167. doi: 10.1073/pnas.64.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson D. M., Steele K., Alexander P. The presence of tumour-specific membrane antigen in the serum of rats with chemically induced sarcomata. Br J Cancer. 1973 Jan;27(1):27–34. doi: 10.1038/bjc.1973.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weir D. M., Elson C. J. Antitissue antibodies and immunological tolerance to self. Arthritis Rheum. 1969 Jun;12(3):254–260. doi: 10.1002/art.1780120314. [DOI] [PubMed] [Google Scholar]
- van Rood J. J., van Leeuwen A., van Santen M. C. Anti HL-A2 inhibitor in normal human serum. Nature. 1970 Apr 25;226(5243):366–367. doi: 10.1038/226366a0. [DOI] [PubMed] [Google Scholar]


