Abstract
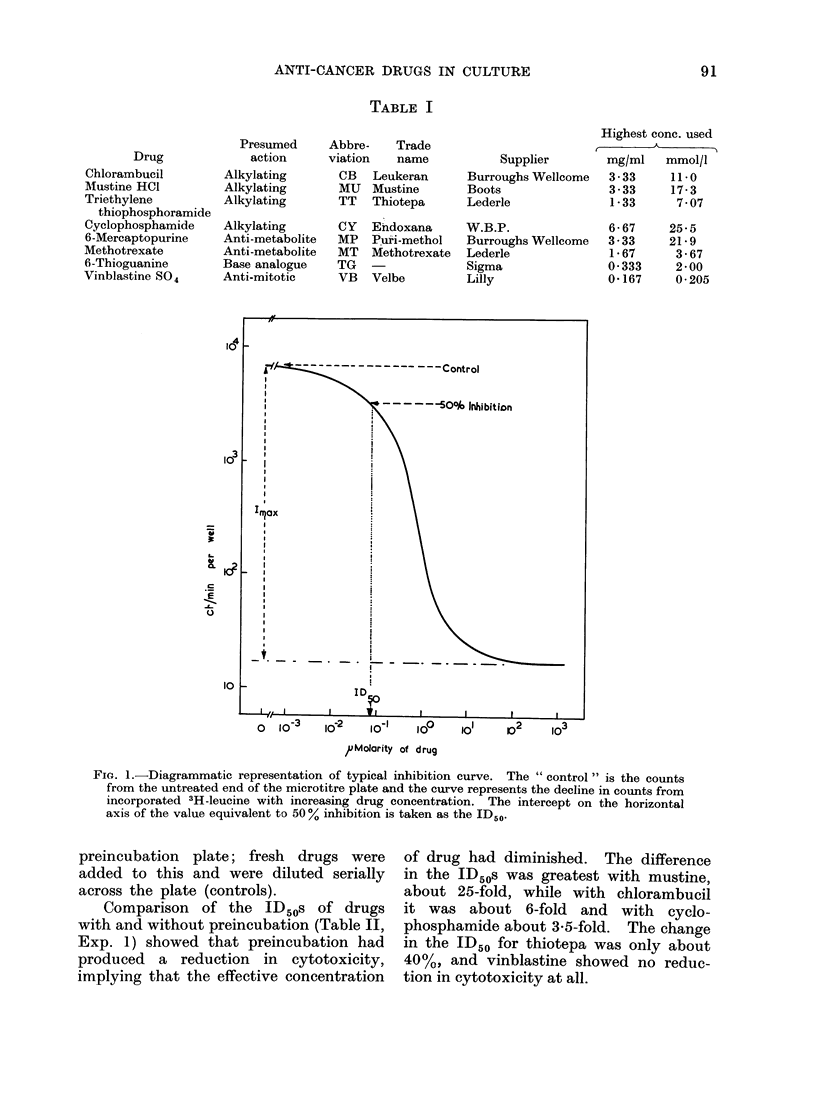
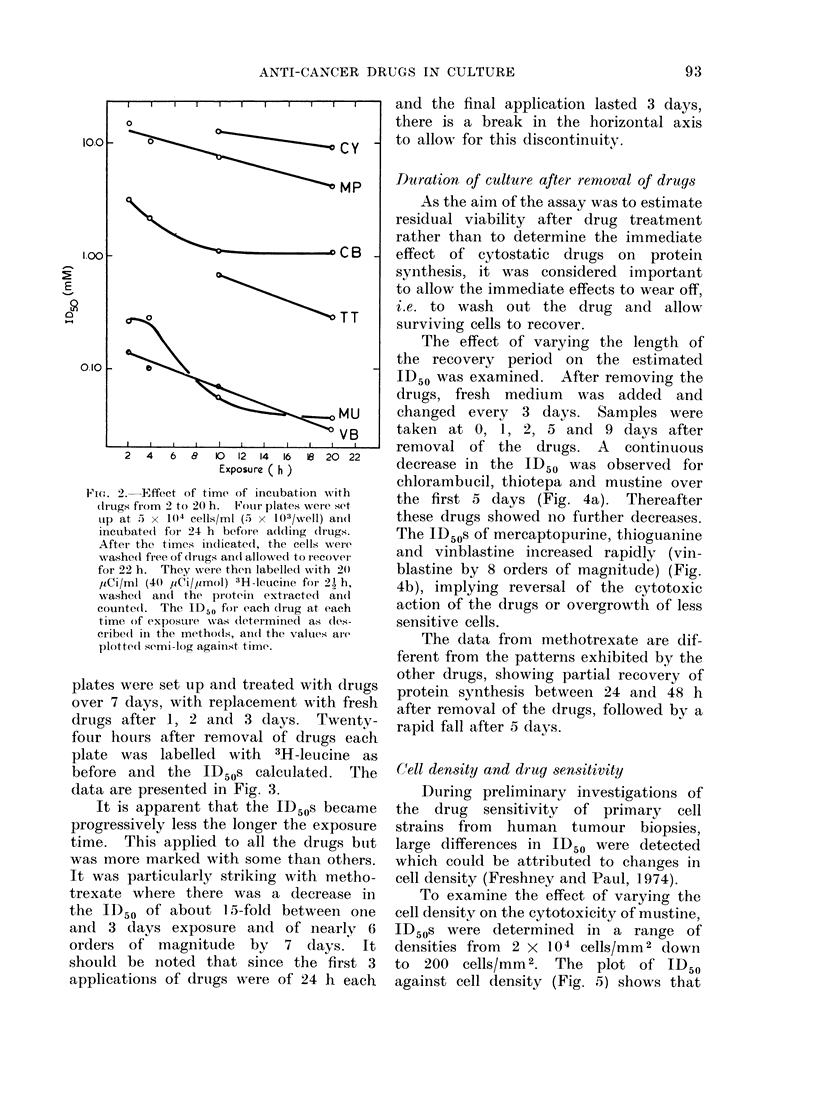
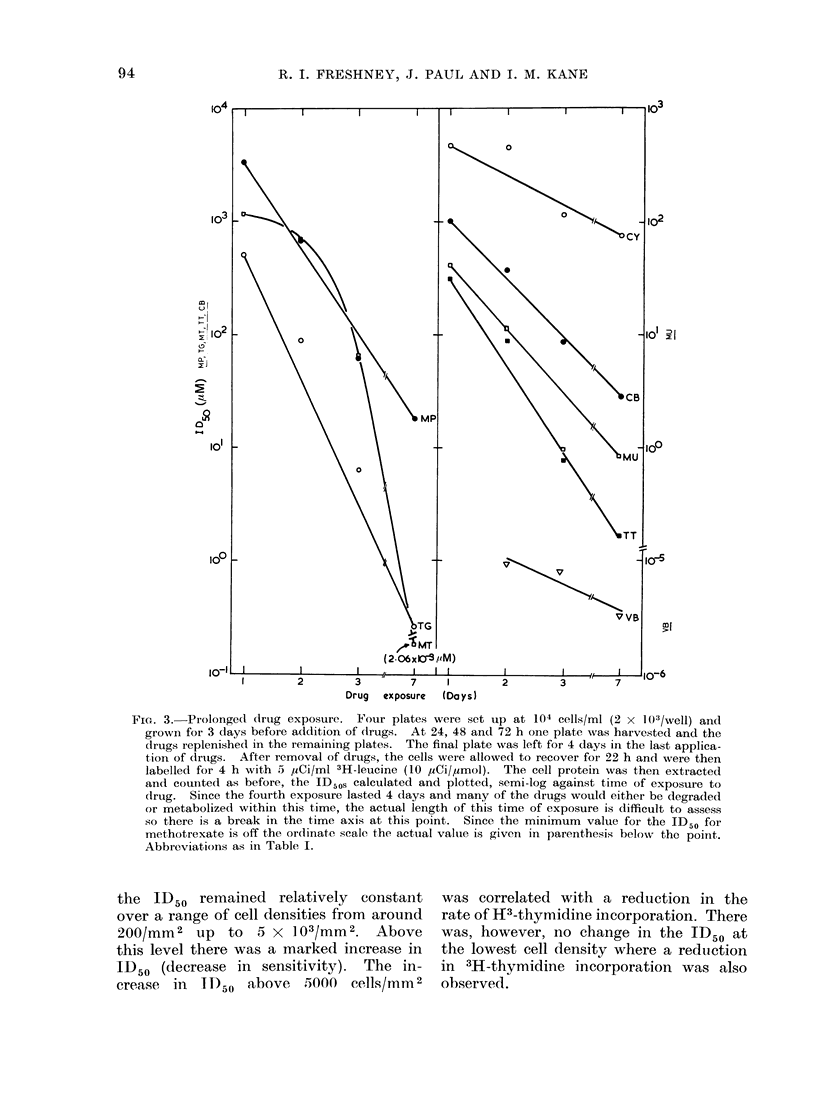
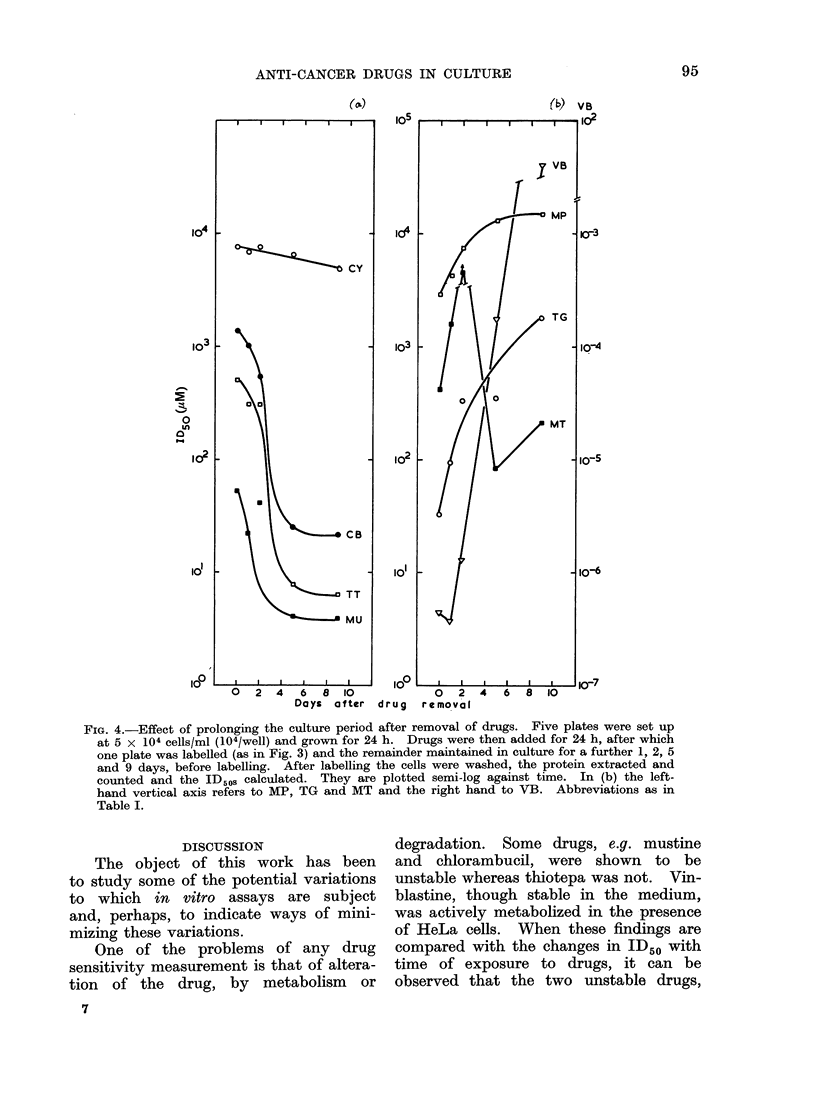
An attempt has been made to construct an assay potentially suitable for use with primary cultures of human tumours to measure the survival of exponentially growing monolayer cultures after exposure to anti-neoplastic drugs. Cell survival was assessed using their protein synthetic capacity after removal of drugs. HeLa cells were employed to avoid the ingerent variability and heterogeneity of primary cultures from human tumours, and an assay has been constructed using microtitration trays to provide large numbers of replicate cultures without the requirement of a large number cells. An increase in the duration of the exposure to drug increased sensitivity in nearly all cases examined. Similarly, an increase in the period of culture following drug removal produced increased sensitivity to alkylating agents but allowed recovery from exposure to certain cycle-dependent drugs. Some of the drugs used were shown to be unstable under culture conditions and vinblastine was actively metabolized, although this instability was not necessarily reflected in the time course of the drug's effect. Mustine sensitivity was shown to be reduced by an increase in cell density at a level where density limitation of 3H-thymidine incorporation becomes apparent. These variations and possible methods of minimizing their effects are discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dolfini E., Martini A., Donnelli M. G., Morasca L., Garattini S. Method for tissue culture evaluation of the cytoxic activity of drugs active through the formation of metabolites. Eur J Cancer. 1973 May;9(5):375–378. doi: 10.1016/0014-2964(73)90054-6. [DOI] [PubMed] [Google Scholar]
- Fox M., Gilbert C. W., Lajtha L. G., Nias A. H. The interpretation of "split-dose" experiments in mammalian cells after treatment with alkylating agents. Chem Biol Interact. 1970 Feb;1(3):241–246. doi: 10.1016/0009-2797(70)90011-6. [DOI] [PubMed] [Google Scholar]
- Goldenberg G. J., Lyons R. M., Lepp J. A., Vanstone C. L. Sensitivity to nitrogen mustard as a function of transport activity and proliferative rate in L5178Y lymphoblasts. Cancer Res. 1971 Nov;31(11):1616–1619. [PubMed] [Google Scholar]
- Hussa R. O., Pattillo R. A. Effects of methotrexate on established cell lines of human choriocarcinoma. Eur J Cancer. 1972 Oct;8(5):523–529. doi: 10.1016/0014-2964(72)90104-1. [DOI] [PubMed] [Google Scholar]
- Limburg H., Heckmann U. Chemotherapy in the treatment of advanced pelvic malignant disease with special reference to ovarian cancer. J Obstet Gynaecol Br Commonw. 1968 Dec;75(12):1246–1255. doi: 10.1111/j.1471-0528.1968.tb02931.x. [DOI] [PubMed] [Google Scholar]
- Morasca L., Balconi G., De Nadai F., Dolfini E. Chemotherapeutic "fingerprints" of two experimental tumors in vitro. Eur J Cancer. 1972 Aug;8(4):429–435. doi: 10.1016/0014-2964(72)90128-4. [DOI] [PubMed] [Google Scholar]
- Ogawa M., Bergsagel D. E., McCulloch E. A. Chemotherapy of mouse myeloma: quantitative cell cultures predictive of response in vivo. Blood. 1973 Jan;41(1):7–15. [PubMed] [Google Scholar]
- Sladek N. E. Bioassay and relative cytotoxic potency of cyclophosphamide metabolites generated in vitro and in vivo. Cancer Res. 1973 Jun;33(6):1150–1158. [PubMed] [Google Scholar]
- Tidd D. M., Kim S. C., Horakova K., Moriwaki A., Paterson A. R. A delayed cytotoxic reaction for 6-mercaptopurine. Cancer Res. 1972 Feb;32(2):317–322. [PubMed] [Google Scholar]


