Abstract
To investigate the impact of chloride (Cl–) permeability, mediated by residual activity of the cystic fibrosis transmembrane conductance regulator (CFTR) or by other Cl– channels, on the manifestations of cystic fibrosis (CF), we determined Cl– transport properties of the respiratory and intestinal tracts in ΔF508 homozygous twins and siblings. In the majority of patients, cAMP and/or Ca2+-regulated Cl– conductance was detected in the airways and intestine. Our finding of cAMP-mediated Cl– conductance suggests that, in vivo, at least some ΔF508 CFTR can reach the plasma membrane and affect Cl– permeability. In respiratory tissue, the expression of basal CFTR-mediated Cl– conductance, demonstrated by 30% of ΔF508 homozygotes, was identified as a positive predictor of milder CF disease. In intestinal tissue, 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid–insensitive (DIDS-insensitive) Cl– secretion, which is indicative of functional CFTR channels, correlated with a milder phenotype, whereas DIDS-sensitive Cl– secretion was observed mainly in more severely affected patients. The more concordant Cl– secretory patterns within monozygous twins compared with dizygous pairs imply that genes other than CFTR significantly influence the manifestation of the basic defect.
Introduction
Cystic fibrosis (CF) is the most common lethal autosomal recessive disease in the Caucasian population, with highly variable manifestations in the pulmonary, gastrointestinal, hepatobiliary, and urogenital tracts (1). It is caused by mutations in the CF transmembrane conductance regulator (CFTR) gene, which encodes a cAMP-regulated chloride (Cl–) channel (2, 3) that is located in the apical membrane of exocrine epithelia. The deletion of a phenylalanine residue at position 508 (ΔF508) is the most frequent of more than 900 known CFTR mutations, and accounts for about 70% of all CF alleles worldwide (4, 5).
The heterogeneity of CF disease is partly explained by the allelic heterogeneity of the CFTR locus. CFTR gene mutations have been categorized by their resulting phenotype into four classes (6). ΔF508 CFTR has been assigned to class II, implying that the protein fails to reach the cell membrane to function as a Cl– channel. This classification was based on earlier experiments done in heterologous model systems and immunocytochemistry of patients’ sweat glands, in which deviant CFTR expression was found in ΔF508 cells (7, 8). Since clinical presentation varies significantly among patients with the same CFTR genotype, and among various affected organs within CF patients, it is evident that factors in addition to the CFTR genotype are involved in determining CF disease severity. In cftr–/– knockout mice, an alternative Ca2+-regulated Cl– conductance was detected in the airways and pancreas; it was suggested that this conductance ameliorates CF lung disease and protects the tissue from the absence of CFTR-mediated Cl– conductance (9). In addition, Ca2+-activated Cl– currents were observed in intestinal tissue of cftr –/– knockout mice with prolonged survival (10). The variable electrophysiological characteristics in human ΔF508 CF respiratory and intestinal tissue (11–13) might be explained by these alternative Ca2+-regulated Cl– channels (14–16).
Furthermore, recent immunocytochemical studies on intestinal, respiratory, and hepatobiliary epithelia of ΔF508 homozygous CF patients have revealed that ΔF508 CFTR may display apical distribution as is seen in control tissues, demonstrating that at least a portion of ΔF508 CFTR can be targeted to the plasma membrane (17, 18). To clarify whether ΔF508 CFTR is competent to transport Cl– across epithelial membranes in vivo, and whether the presence of Cl– transport can influence CF disease, the relative contribution of ΔF508 CFTR and alternative Cl– channels needs to be assessed in patients’ tissues in correlation to the CF phenotype.
In this study, we investigated the basic Cl– secretory defect in the most severely affected tissues in CF, the respiratory and intestinal tracts. Subjects were CF twins and siblings homozygous for the ΔF508 CFTR gene mutation. We determined the bioelectrical properties of the tissues using the nasal potential difference (NPD) measurement and the intestinal current (IC) measurement, respectively. These methods assess the cAMP and Ca2+-induced Cl– secretory pathways by adding specific secretagogues and inhibitors, with which the presence of CFTR and/or alternative Cl– transporters can be determined. The influence of Cl– permeability on clinical CF phenotype in specific organs was evaluated by determining the presence and origin of different Cl– conductances in subgroups of ΔF508 homozygous CF twin and sibling pairs with disparate manifestation of CF disease. These individuals with the most extreme clinical parameters are highly informative in the investigation of factors influencing the CF phenotype (19–21).
The investigation of ΔF508 homozygous twins and siblings is the classical approach for analyzing a monogenic disease like CF, and for differentiating the relative importance of the major genetic lesion and other genetic and environmental factors (22, 23). The disease-causing CFTR mutation and the intragenic haplotype are standardized, and the variation in genetic background is either reduced (in dizygous pairs) or eliminated (in monozygous twins). Dizygous twins and siblings share on average half of their genes. The intrapair variation among monozygous twins, who are genetically identical in their entire genome, can be used to delineate the relative contribution of genetic and environmental factors to a trait by comparing the intrapair variability among monozygous pairs to that of dizygous pairs. CF twins and siblings share many environmental factors that are major determinants of CF disease severity, e.g., physician, therapeutic regime, living conditions, and behavioral patterns. Thus, the influence of genetic background and environmental factors on the genotype-phenotype correlation can be examined in greater depth in these subjects than in a cohort of unrelated CF patients.
Methods
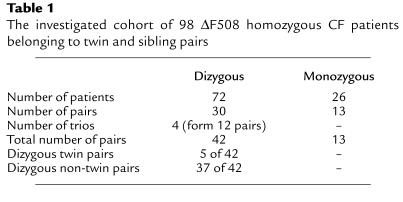
Subjects.
The investigated ΔF508 homozygous patient pairs were recruited from a set of 114 CF monozygous and dizygous pairs recruited by European physicians to enroll in the European Cystic Fibrosis Twin and Sibling Study. Out of these 228 ΔF508 homozygous patients, a cohort of 98 patients (54 females, 44 males) was actually able to take part in the clinical assessment and the IC and/or NPD measurements. These 98 patients belonged to 43 pairs and 4 trios. For data comparisons, each trio was treated as 3 separate sibling pairs, resulting in a total of 55 pairs (Table 1). Clinical assessments and NPD and IC measurements were carried out at CF clinics in Hannover (22 pairs), Innsbruck (6 pairs), London (5 pairs), Rotterdam (18 pairs), and Verona (4 pairs). Mono- or dizygosity of twins was ascertained by using the AmpFLSTR Profiler Plus PCR amplification kit, analyzed on the ABI Prism 377 DNA sequencer (both from Perkin-Elmer Ltd., Beaconsfield, United Kingdom) (24), or by oligonucleotide fingerprinting of simple repeats applying in situ gel hybridization of MboI or Hinf I genomic digests (25). Thirteen of the 55 pairs were monozygous twin pairs, 5 pairs were dizygous twin pairs, and there were 37 dizygous non-twin pairs (Table 1). Homozygosity for ΔF508 in patients, and heterozygosity for ΔF508 in parents were verified by heteroduplex analysis (26). Clinical data of the complete cohort of patients participating in this study are provided in Table 2. Patients were not experiencing an episode of allergic bronchopulmonary aspergillosis or taking any systemic corticosteroids on the day of investigation, either of which could influence the performed measurements. Liver disease was determined in 1 pair. Three patients, all belonging to different pairs, were being treated with insulin for diabetes mellitus. All participating patients were pancreatic insufficient, as ascertained by the stool elastase test. Performed examinations were approved by the medical ethics committees of the local hospitals and by patients or parents by written informed consent.
Table 1.
The investigated cohort of 98 ΔF508 homozygous CF patients belonging to twin and sibling pairs
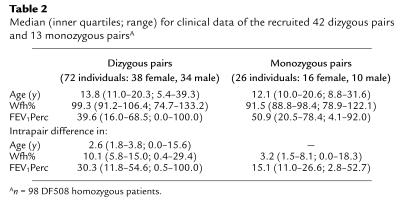
Table 2.
Median (inner quartiles; range) for clinical data of the recruited 42 dizygous pairs and 13 monozygous pairsA
Assessment of clinical phenotype.
In order to select the most extreme phenotypes of the patient pairs, overall disease severity and intrapair discordance were quantified as reported (27). Briefly, a patient’s nutritional status was characterized by the anthropometric parameter weight, expressed as predicted weight for height percentage (wfh%), using the data published by Prader et al. (28). Pulmonary status was assessed by forced expiratory volume in 1 second (FEV1), expressed as a predicted value (FEV1%pred) based on the formula by Knudson et al. (29). FEV1%pred declines with age as expected for progressive lung disease in CF (30). Therefore, we used age-specific percentiles for FEV1%pred (FEV1Perc), calculated from the CF population database published in the report of the European Epidemiological Registry of Cystic Fibrosis (31),to correct for the age-related decline in FEV1%pred in our cohort. Thus, wfh% and FEV1Perc, two of the most sensitive age-independent parameters for course and prognosis, were evaluated to characterize CF disease severity in the two major afflicted organs in CF, the gastrointestinal and respiratory tracts.
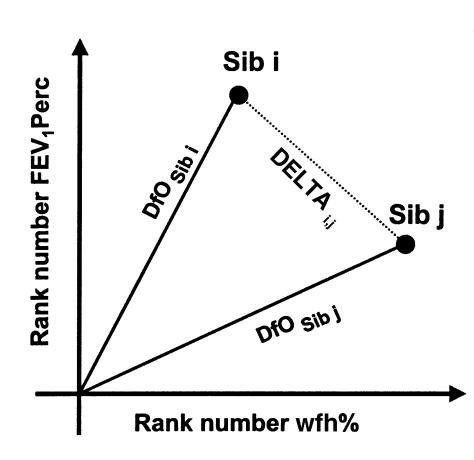
The CF patient pairs with the most disparate phenotypes, i.e., the most informative pairs, were selected as follows (27): First, rank numbers for wfh% (x) and FEV1Perc (y) were assigned to the complete cohort, whereby rank number 1 identified the most severely affected patient (Figure 1). The disease severity of patient i was determined by the distance from origin (DfO) in the plot resulting from the rank numbers xi and yi (Figure 1). Intrapair discordance was defined by the distance (DELTA) between the two data points, representing patients i and j of a pair (Figure 1). Thus, DfO and DELTA were defined by: DfOi = (xi2 + yi2)1/2, and DELTAi,j = [(xi – xj)2 + (yi – yj)2]1/2. With these two parameters, a computer-assisted algorithm was used (27) to segregate patient pairs composed of 2 siblings with both equally high wfh% and equally high FEV1Perc (concordant/mildly affected; CONmild), pairs consisting of 2 siblings with both equally low wfh% and equally low FEV1Perc (concordant/severely affected; CONsevere), and patient pairs comprised of 1 sibling with high wfh% and high FEV1Perc and 1 sibling with low wfh% and low FEV1Perc (discordant pairs; DISC). CONmild pairs are characterized by a low DELTA, but the sum of both DfOs (ΣDfO) is large because the rank numbers for wfh% and FEV1Perc for both siblings are high (Figure 1). CONsevere pairs also display a low DELTA, but ΣDfO is small because DfOs for both siblings are close to the origin. DISC pairs are discriminated by the highest values for DELTA. Other pairs were excluded from these extreme phenotypes by the ranking algorithm.
Figure 1.
Definition of composite parameters DfO and DELTA, which describe overall disease severity and intrapair discordance, respectively. As indicated, DfO and DELTA are determined by the rank numbers for FEV1Perc and wfh% of siblings i and j of a sibling pair.
NPD measurements.
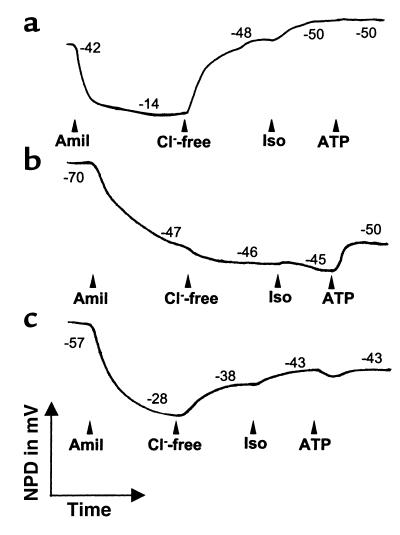
The method of studying NPD has been adapted from a method described previously (32), and measures Cl– and Na+ conductances as potential differences (PDs). In short, the reference bridge was formed by a subcutaneous needle in the forearm, which is isoelectric with the submucosal space of the nasal epithelium. The exploring bridge was positioned under the inferior nasal turbinate to apply the different perfusion solutions. Both the reference bridge and the exploring catheter were connected to a high-input resistance voltmeter via 4% agar-salt bridges and Ag/AgCl electrodes. The basal PD was measured during superfusion of the nasal epithelium with a salt solution (1.7 ml/min) and was found to be lumen-negative with respect to the submucosal reference electrode. The salt solution consisted of (in mmol/l): 120 NaCl, 25 sodium gluconate, 5 potassium gluconate, 0.4 NaH2PO4, and 2.4 Na2HPO4. On the inferior turbinate, the spot with the maximal (most negative) stable baseline PD was selected. This baseline PD was shown to be considerably more negative in CF patients than in non-CF individuals (Figure 2). At this site, NPDs were measured in response to superfusion with solutions of different ion compositions, or containing different drugs (Figure 2). First, the catheter was perfused with amiloride (10–4 mol/l), a blocker of epithelial Na+ channels, thereby inhibiting the PD by eliminating the contribution of electrogenic sodium absorption (33). To determine the basal Cl– conductance, Cl– was replaced with gluconate in the solution containing amiloride. Subsequently, the β-adrenergic agonist isoprenaline (10–4 mol/l) was added to the Cl–-free solution containing amiloride, which induces cAMP-dependent Cl– conductance and determines the presence of CFTR (32). For the last perfusate, ATP (10–3 mol/l) was added to the Cl–-free solution containing amiloride and isoprenaline. ATP binds to purinergic receptors on the luminal surface, triggering the Ca2+-mediated Cl– secretory pathway by activating phospholipase C and increasing intracellular Ca2+ (34). Superfusion of the nasal epithelium with the different perfusates was continued until a steady state was reached, or for at least 3 minutes. In each patient, NPD measurements were performed in the left and right nostrils. The NPD tracing of the nostril with the highest Cl– secretory responses, i.e., with the largest capacity to transport Cl–, was assessed for the evaluations and calculations performed in this study. Tracings of patients with chronic rhinitis or a cold on the day of investigation were discarded from further evaluation (Table 3).
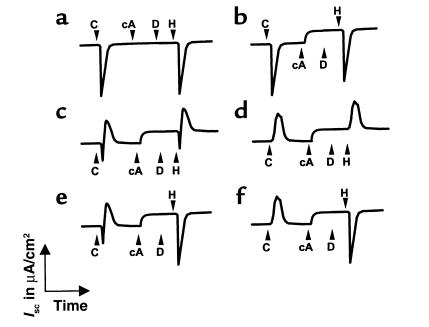
Figure 2.
NPD measurements. Basal PD is measured with saline solution, after which the nasal epithelium is superfused with amiloride, Cl–-free solution (gluconate substituted for Cl–), isoprenaline, and ATP. Tracings are shown for (a) non-CF control, (b) CF patient without a gluconate or isoprenaline response, but with a response to ATP, (c) CF patient exhibiting chloride conductance initiated by superfusion with the Cl–-free solution and by the solution containing isoprenaline. Amil, amiloride; Iso, isoprenaline.
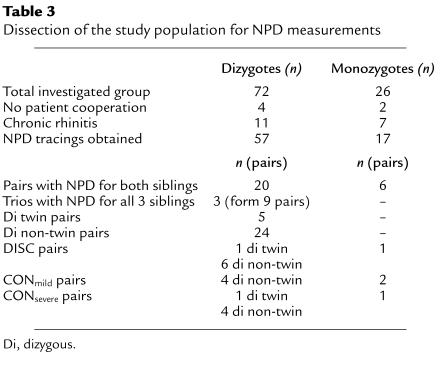
Table 3.
Dissection of the study population for NPD measurements
IC measurements in rectal biopsies.
The method used to study IC has been described previously (11, 12, 35). It determines Na+ and Cl– fluxes in the intestinal epithelium as a change in short-circuit current (ΔIsc). Freshly obtained rectal suction biopsies were mounted in adapted micro-Ussing chambers with an aperture of 1.2 mm (35). The tissue was perfused with buffer solution at 37°C (composition in mmol/l: 126.2 Na+, 114.3 Cl–, 20.2 HCO3–, 0.3 HPO42–, 0.4 H2PO4–, and 10 HEPES; pH 7.4) and gassed with 95% O2 and 5% CO2. Basal transepithelial resistance was determined by the voltage response to pulse currents of 1 μA and application of Ohm’s law. Basal Isc prior to voltage clamping was calculated from the basal transepithelial resistance and the open-circuit transepithelial PD. Subsequently, the tissue was short-circuited using voltage clamps for the course of the experiment. For cell metabolism, glucose (10–2 mol/l) was given both mucosally and serosally. After equilibration, the following pharmaceuticals were added in a standardized order to the mucosal (M) and/or serosal (S) side: (a) amiloride (10–4 mol/l, M) (33); (b) indomethacin (10–5 mol/l, M + S), to reduce basal Cl– secretion by inhibiting the endogenous prostaglandin formation (36); (c) carbachol (10–4 mol/l, S), to initiate the cholinergic Ca2+-linked Cl– secretion (37); (d) forskolin (10–5 mol/l, M + S) (38) together with 8-bromo-cAMP (10–3 mol/l, M+S), to open cAMP-dependent Cl– channels such as CFTR and the outwardly rectifying Cl– channel (ORCC) (39); (e) 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS; 2.10–4 mol/l, M), to inhibit DIDS-sensitive Cl– transporters like the Ca2+-dependent Cl– channels (14, 15) and the ORCC (39); and (f) histamine (5.10–4 mol/l, S), to reactivate the Ca2+-dependent Cl– secretory pathway (40). Two rectal biopsies were obtained for every patient participating in the IC measurements. The IC tracing belonging to the biopsy with the highest Cl– secretory responses was included in the computations. All drugs for NPD and IC measurements were obtained from Sigma Chemical Co. (St. Louis, Missouri, USA).
Interpretation of IC measurement patterns.
By using the above-mentioned IC measurement protocol, at least six different IC measurement patterns could be observed in tracings from CF individuals (Figure 3). To quantify the Cl– secretory responses to carbachol and histamine, the net change in Isc was defined as the sum of the negative downward peak below baseline, and the positive upward peak above baseline in the Cl– secretory direction. A net negative response points to no or little Cl– secretion, whereas a net positive change in Isc indicates a high amount of Cl– secretion. The influence of DIDS on Cl– secretion was determined by comparing the net Cl– secretory response to carbachol before the addition of DIDS, to the net Cl– secretory response to histamine after incubation with DIDS (12).
Figure 3.
Examples of IC response patterns observed in ΔF508 homozygous CF patients upon the addition of carbachol, 8-bromo-cAMP + forskolin, DIDS, and histamine. A downward, negative peak upon addition of carbachol or histamine is indicative of K+ efflux unmasked in the absence of Cl– secretion (50), while an upward peak corresponds to Cl– secretion. (a) Tracing of a typical CF patient showing no response in the Cl– secretory direction to any of the applied compounds. (b) cAMP-mediated residual Cl– secretion initiated only by cAMP + forskolin; these are more sensitive indicators for cAMP-mediated Cl– secretion than carbachol or histamine (11, 12). (c and d) DIDS-insensitive residual Cl– secretion initiated not only by cAMP but by carbachol and histamine as well. Responses range from a peak in the reverse direction followed by a small change in Isc in the Cl– secretory direction (c), to responses that consist purely of a subnormal Cl– secretory peak (d). (e and f) DIDS-sensitive residual Cl– secretion in the presence of a cAMP response: carbachol causes a Cl– secretory response that does not occur with histamine response since Cl– secretion is inhibited by DIDS. C, carbachol; cA, 8-bromo-cAMP + forskolin; D, DIDS; H, histamine.
Statistical analysis.
The statistical analysis of our results was performed using the Mann-Whitney U test, the Student t test (for grouped pairs), Fisher’s exact test, and Spearman’s rank correlation test. P values less than or equal to 0.05 were considered statistically significant.
Results
NPD measurements.
Of the 98 ΔF508 homozygous patients, we obtained NPD data for 57 individuals belonging to dizygous pairs and 17 individuals of monozygous pairs (Table 3). NPD tracings of 11 individuals belonging to dizygous pairs and of 7 individuals belonging to monozygous pairs with respiratory inflammation were not included in the analysis. Four patients of dizygous pairs and 2 patients of monozygous pairs did not accept or endure the procedure for the NPD measurements. In the set of 57 NPD recordings of dizygous individuals, 20 pairs were represented in addition to 3 trios forming 9 pairs (Table 3). Of these 29 pairs, 24 were dizygous non-twin pairs, and 5 pairs were dizygous twin pairs. For 6 monozygous pairs, NPD tracings were obtained for both sibling A and B of a pair. The patient pairs were categorized into one of three extreme phenotypes (DISC, CONmild, and CONsevere) according to the ranking algorithm using wfh% and FEV1Perc as described in Methods. A total of 16 dizygous non-twin and dizygous twin pairs and 4 monozygous twin pairs fit the criteria of one of the phenotypic groups DISC, CONmild, or CONsevere (Table 3).
In our group of ΔF508 homozygous patients, neither the absolute age, the intrapair difference in age, nor the sex of individuals was associated with different outcomes in NPD measurements. Hence, neither age nor sex influenced intra- or interpair differences in NPD responses observed between the dizygous and monozygous pairs, or between the three phenotypic groups (data not shown).
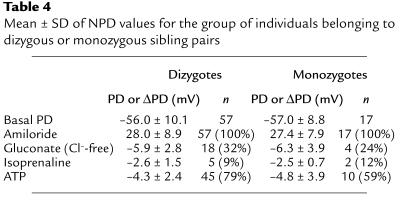
The basal PD measured in 57 individuals belonging to dizygous sibling pairs was –56.0 ± 10.1 mV, and the decrease in PD in response to amiloride was 28.0 ± 8.9 mV (Table 4). In 17 patients belonging to monozygous twin pairs, the basal PD was –57.0 ± 8.8 mV, while the amiloride response was 27.4 ± 7.9 mV. Eighteen individuals belonging to dizygous sibling pairs exhibited small amounts of basal Cl– conductance, measured by superfusion with a Cl–-free solution (Table 4), while 5 persons demonstrated Cl– conductance initiated by the addition of isoprenaline, and 45 patients reacted to the addition of ATP. The PD of 4 individuals of monozygous twin pairs increased in response to superfusion with Cl–-free solution, while increases were seen in 2 individuals when isoprenaline was added, and in 10 persons upon addition of ATP. This shows that the distribution of PD responses was independent of zygosity (Table 4).
Table 4.
Mean ± SD of NPD values for the group of individuals belonging to dizygous or monozygous sibling pairs
To assess the correlation between the basic defect and the phenotype in the respiratory tissue, NPD results measured within the three subgroups of extreme phenotypes (DISC, CONmild, and CONsevere) were compared with the lung function parameter FEV1Perc.
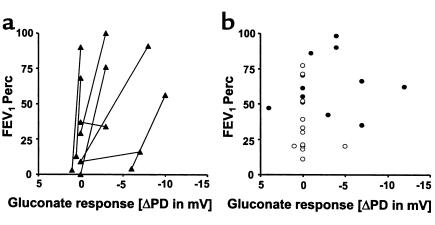
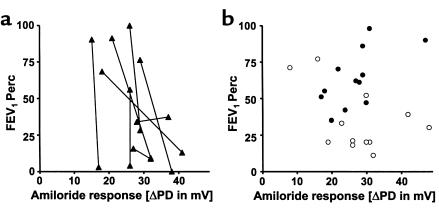
The lung functions of 8 DISC sibling pairs were plotted against their responses to superfusion with Cl–-free solution (Figure 4a). The siblings with the better lung function parameters demonstrated significantly higher (more negative) gluconate responses than their paired siblings did (P < 0.05, Student t test for grouped pairs), and also possessed the better wfh% of the 2 siblings (data not shown). When the lung function of patients belonging to CONmild and CONsevere pairs was plotted against their gluconate responses, all persons (except 1) who had a gluconate response (i.e., increased negativity) belonged to CONmild pairs (Figure 4b). The presence of this Cl– secretory response upon addition of gluconate was significantly associated with the CONmild phenotype (P = 0.013, Fisher’s exact test). Only 1 patient belonging to a CONsevere pair showed a response to gluconate. Similarly, no responses to isoprenaline were observed in severely affected patients, i.e., patients in CONsevere pairs or the severely affected siblings of DISC pairs.
Figure 4.
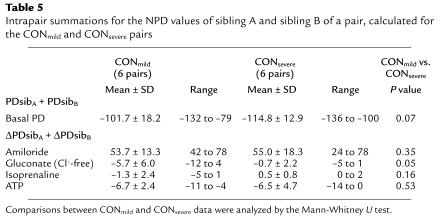
FEV1Perc versus the change in NPD upon addition of a Cl–-free solution (gluconate substituted for Cl–) for 8 pairs that were categorized DISC for the FEV1Perc parameter (a). The 8 pairs consisted of 1 dizygous twin, 6 dizygous non-twin pairs, and 1 monozygous twin pair (Table 3). The sibling with the higher (more negative) response to gluconate within a pair was also the sibling with the higher lung function (P < 0.05, Student t test for grouped pairs). (b) FEV1Perc versus the gluconate response for 6 pairs that were ranked as CONmild (filled circles) (4 dizygous non-twin and 2 monozygous twin pairs), and 6 pairs who were ranked as CONsevere (open circles) (1 dizygous twin, 4 dizygous non-twin, and 1 monozygous twin pair). A hyperpolarization of the PD in response to gluconate, pointing to the presence of Cl– conductance, segregated with the CONmild pairs (P = 0.013, Fisher’s exact test).
The amiloride-induced depolarization observed in all patients (Figure 5), and the ATP-induced hyperpolarization (increased negativity) of PD seen in 55 of the 74 recorded NPD tracings, did not show any significant correlations with lung function.
Figure 5.
FEV1Perc versus the change in NPD upon addition of amiloride. (Patient pairs and symbols as shown in Figure 4.) Comparisons between lung function and the magnitude of the amiloride response were not significant (Student t test for grouped pairs) for (a) the 8 DISC pairs (P < 0.1), or (b) the 6 CONmild (P = 0.41) and 6 CONsevere pairs (P = 0.33).
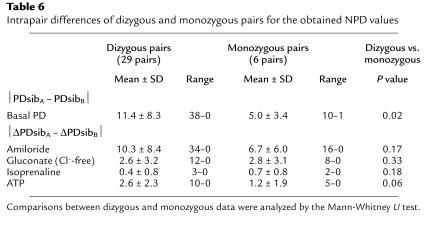
Substantiation of the observed associations between NPD values and phenotype was provided by the following results: For each pair, the NPD values of the individual siblings of a pair were added, corresponding to PDsibA + PDsibB in the case of the basal PD, and ΔPDsibA + ΔPDsibB for the responses to the different solutions applied to the nasal epithelium (Table 5). Summations calculated for the CONmild pairs were compared with those of the CONsevere pairs (Table 5). The intrapair summations for the basal PDs of the CONmild pairs were less negative than those for the CONsevere pairs (–101.7 ± 18.2 versus –114.8 ± 12.9); however, this was not significant (Table 5; P < 0.10, Mann-Whitney U test). The ΔPDsibA + ΔPDsibB values for the gluconate responses were significantly more negative for the CONmild pairs (P < 0.05), while ΔPDsibA + ΔPDsibB values for the amiloride and ATP responses did not differ between CONmild and CONsevere pairs. The ΔPDsibA + ΔPDsibB values for the isoprenaline responses within CONmild and CONsevere pairs were not significantly different; most investigated individuals had no response, and in patients who did respond to isoprenaline, the magnitude of the response was small. However, ΔPDsibA + ΔPDsibB for isoprenaline was negative in the CONmild couples (–1.3 ± 2.4) and positive in the CONsevere couples (0.5 ± 0.8). As mentioned above, no Cl– conductance was induced by isoprenaline in siblings of CONsevere pairs.
Table 5.
Intrapair summations for the NPD values of sibling A and sibling B of a pair, calculated for the CONmild and CONsevere pairs
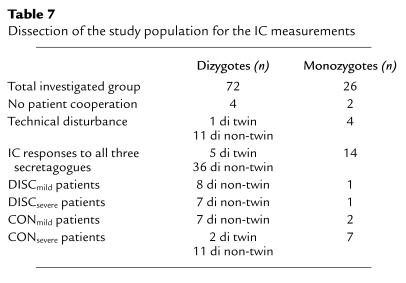
To analyze the intrapair concordance in NPD values of monozygous pairs versus dizygous pairs, intrapair differences were calculated by the absolute values ¦PDsibA – PDsibB¦ for the basal PD, and ¦ΔPDsibA – ΔPDsibB¦ for responses to applied substances (Table 6). Monozygous twins (5.0 ± 3.4 mV) were more concordant than dizygous pairs (11.4 ± 8.3 mV, P < 0.05) in baseline PD, but not in any other parameter (Table 6).
Table 6.
Intrapair differences of dizygous and monozygous pairs for the obtained NPD values
IC measurements.
For various reasons, IC measurements were not obtained for all 98 ΔF508 homozygous individuals (Table 7 and ref. 12). Patients who had interpretable responses to all three secretagogues (carbachol, 8-bromo-cAMP, and histamine) and who belonged to a pair that could be assigned to one of the extreme phenotypes (DISC, CONmild, and CONsevere) were included in the analyses of this study. To avoid the loss of IC data points, we included results from pairs in which both siblings had complete IC measurement results, as well as IC measurements from pairs in whom only 1 sibling produced a complete IC recording. Table 7 indicates the number of patients matching the criteria of the different phenotypic groups with complete IC tracings.
Table 7.
Dissection of the study population for the IC measurements
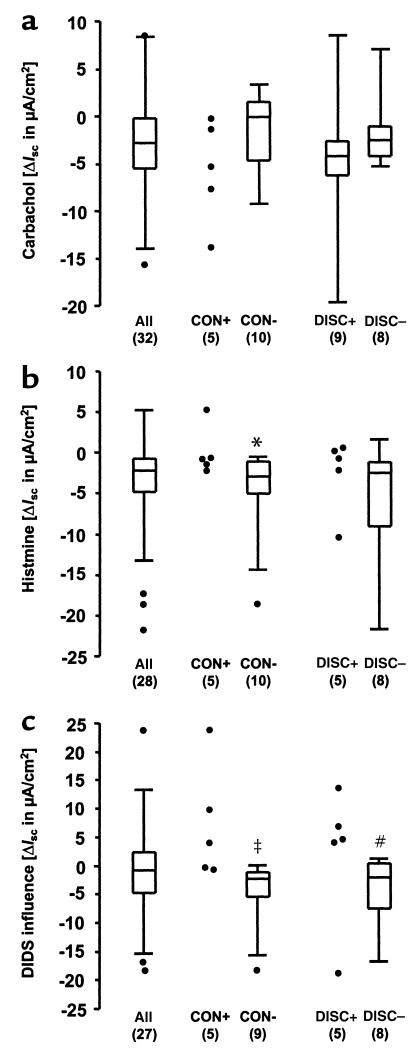
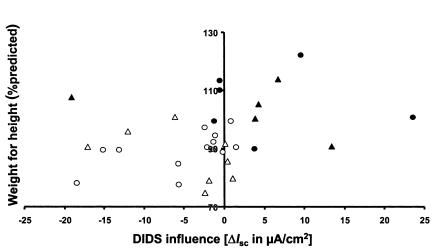
To assess the impact of intestinal Cl– secretion on clinical outcome, responses to the application of carbachol and histamine, and the influence of DIDS on Cl– secretory responses were compared in the CONmild, CONsevere, and DISC groups (Figure 6). Carbachol responses within the mildly affected siblings, i.e., siblings belonging to CONmild pairs or the less affected siblings of DISC pairs, were not different from responses in CONsevere pairs or the sicker siblings of DISC pairs (Figure 6a). Histamine responses, however, were less negative in the mildly affected siblings, which was significant when siblings of CONmild and CONsevere pairs were compared (Figure 6b; P = 0.05, Mann-Whitney U test). In addition, the difference in carbachol and histamine responses due to the presence of DIDS was significant, both when CONmild and CONsevere siblings were compared (0.001 < P < 0.01) and when DISCmild and DISCsevere siblings were compared (Figure 6c; P = 0.05). Additionally, wfh% was positively correlated to the magnitude of the DIDS influence on Cl– secretion (Figure 7; P < 0.025, Spearman’s test).
Figure 6.
Net Isc responses to carbachol (a) and histamine (b), and the influence of DIDS on the Cl– secretory response (c). Results are given for the whole group (all), and for the siblings belonging to CONmild, CONsevere, and DISC pairs. For the CONmild and CONsevere pairs, individuals were counted when there was a measurement value for only 1 sibling of a pair, while the mean of both siblings was included when there was a measurement for both siblings. For the individuals belonging to DISC pairs, data are compared between the clinically better (DISCmild) and worse (DISCsevere) siblings. For each group of data, the interquartile range (IQR) is depicted by a rectangle, with the upper horizontal line indicating the 75th percentile, followed by the 50th percentile, and the lower horizontal line presenting the 25th percentile. The spread for the rest of the data points in the group is given by the upper and lower vertical lines initiating from the rectangle. Data points larger than 75th percentile + 1.5 IQR, and data points smaller than 25th percentile – 1.5 IQR are considered outliers and are indicated as individual data points. For groups with n < 6, single data points are given. Statistical comparisons were performed by the Mann-Whitney U test. *P ≤ 0.05 vs. CONmild, ‡0.001 < P < 0.01 vs. CONmild, #P ≤ 0.05 vs. DISCmild. CON+, CONmild; CON–, CONsevere; DISC+, DISCmild; DISC–, DISCsevere.
Figure 7.
Wfh% plotted against the influence of DIDS on the Cl– secretory response, calculated as the difference between the carbachol response before the incubation of the tissue with DIDS, and the histamine response in the presence of DIDS. Values are plotted for siblings of CONmild (filled circles) pairs, CONsevere (open circles) pairs, and the mildly affected and severely affected siblings of DISC pairs (DISCmild, filled triangles; and DISCsevere, open triangles). All CONmild and DISCmild siblings except one exhibited either a small negative or even a positive influence of DIDS, meaning that DIDS did not inhibit Cl– secretion in these individuals. In contrast, DIDS inhibited the majority of Cl– secretory responses of the CONsevere and DISCsevere siblings. This negative DIDS influence correlated significantly with the CONsevere and DISCsevere patients (P = 0.03, Fisher’s exact test). Moreover, the magnitude of wfh% correlated with the magnitude of the DIDS influence (P < 0.025, Spearman’s test).
As previously reported (12), monozygous twins were more concordant in their IC patterns than dizygous pairs were, which was significant in the cAMP-stimulated Cl– secretory responses, the histamine responses, and the influence of DIDS on Cl– secretory responses.
NPD and IC measurements.
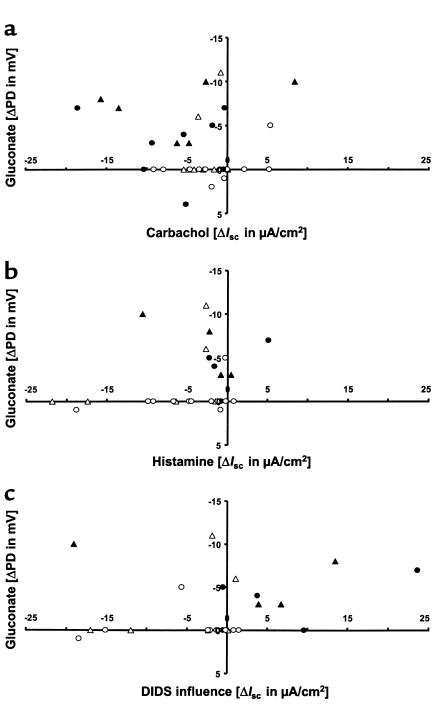
To assess differences and/or similarities in the presence and magnitude of Cl– conductances between respiratory and intestinal tissues, the NPD results were compared with the IC results. The presence of a gluconate response did not correspond to the presence of a Cl– secretory component in the carbachol and/or histamine response, and there were no significant correlations between the amplitude of the gluconate response and the amplitudes of the carbachol and histamine responses (Figure 8, a and b). However, in this preselected cohort of extreme phenotypes, the presence of a gluconate response in the respiratory tissue and a positive DIDS influence in the intestinal tissue corresponded with a milder phenotype (Figure 8c; P = 0.01, Fisher’s exact test). Moreover, the magnitude of the gluconate response was related to the magnitude of the DIDS influence on Cl– secretion (Figure 8c; Spearman’s test, reaching the limit of significance).
Figure 8.
Cl– conductance in respiratory tissue measured by the gluconate response, compared with Cl– secretion in intestinal tissue. Values are given for individuals belonging to CONmild (filled circles) and CONsevere (open circles) pairs, and for DISCmild siblings (filled triangles) and DISCsevere siblings (open triangles). The gluconate response measured in NPD plotted versus the Cl– secretory response observed in ICs upon the addition of (a) carbachol or (b) histamine, and (c) versus the influence on Cl– secretion exerted by DIDS. While the gluconate response did not correlate with the carbachol or histamine responses, the magnitude of the gluconate response was associated with the magnitude of the DIDS influence (0.05 < α < 0.1, non-significant trend, Spearman’s test). A negative gluconate response pointing to Cl– conductance in combination with a positive DIDS influence correlated with the CONmild and DISCmild siblings (P = 0.01, Fisher’s exact test).
Discussion
In this study, we sought to determine whether the basic defect in CF, which presents itself in the form of aberrant Cl– transport properties, is predictive of the clinical outcome in CF, and if manifestation of the basic defect is substantially determined by inherited factors apart from the CFTR gene. The observation that disease expression in CF is highly heterogeneous even in patients with the same CFTR mutation genotype indicates that factors other than the CFTR gene itself contribute to the course of CF disease. The approach of the European Cystic Fibrosis Twin and Sibling Study, of investigating mono- and dizygous ΔF508 homozygous pairs with the extreme CF phenotypes, is the optimal scenario for investigating the relative impact of environmental factors and/or inherited factors other than the CFTR gene (19–23).
Our results demonstrate that many of the ΔF508 homozygous CF individuals express some Cl– channel activity in the respiratory tract, as we reported earlier for the intestinal tract (11, 12). In those patients with apparent cAMP-mediated Cl– conductance, i.e., an isoprenaline response and/or a DIDS-insensitive Cl– secretory response, the molecular entity is most likely CFTR, indicating the presence of functional ΔF508 CFTR in the apical membranes of the epithelial cells in these organs. On the basis of studies in heterologous expression systems (7, 8), the ΔF508 CFTR gene mutation is considered to be a processing defect involving reduced glycosylation and consequent misfolding of the CFTR protein, which prevents the protein from reaching the plasma membrane and causes it to be retained in the endoplasmic reticulum. However, the expression of ΔF508 CFTR in vivo might be different. Immunohistochemical studies performed on airway, intestinal, and hepatobiliary tissues (17, 18, 41, 42) have demonstrated ΔF508 CFTR localization in the plasma membrane. Moreover, functional assays of transfected cells (43), in mice (44, 45), and of human tissue specimens (18) have shown that ΔF508 CFTR is capable of transporting Cl– in response to cAMP agonists. Our in vivo and ex vivo studies show that respiratory and intestinal tissues from ΔF508 CFTR homozygous individuals are competent to respond to agonists of the cAMP-dependent Cl– secretory pathway, which is the hallmark of CFTR-mediated Cl– transport (2, 3). Nonetheless, the magnitude of this detected Cl– permeability is evidently insufficient to prevent manifestation of CF disease.
Our data confirm a correlation between better lung function and less anomalous bioelectrical properties of the nasal epithelium in the investigated ΔF508 homozygotes. The respiratory epithelium of healthier CF patients appears to be more permeable to Cl– than that of sicker patients (Figure 4, a and b). Moreover, the presence of basal Cl– conductance and the capacity to secrete Cl– in response to a cAMP agonist, determined by perfusion of the nasal epithelium with a Cl–-free solution and isoprenaline, respectively, were present only in mildly affected patients (Table 4 and Figure 4). This suggests that the expression of basal Cl– conductance and/or residual cAMP-mediated Cl– conductance has a beneficial influence on respiratory tissue function, most likely by increasing the hydration of the viscous airway surface mucus and increasing its clearance from the respiratory tract. These data from ΔF508 homozygous twins and siblings substantiate the findings in an earlier study of CF individuals with different CFTR mutation genotypes, in whom the expression of Cl– conductance provided a better indication of lung function than did genotype (13). In contrast, Cl– conductance in the amiloride-pretreated nasal epithelium that is mediated by apical purinergic receptors and a subsequent increase of intracellular Ca2+ (34) was not associated with better preservation of tissue function (Table 5; P = 0.53). Similarly, as inferred from the amiloride response, the increased Na+ absorption in nasal epithelium that is characteristic for CF (46) and has been suggested to contribute to pulmonary disease (47, 48) was not directly related to CF disease severity (Figure 5).
In our group of investigated ΔF508 homozygous individuals, the correlation between the isoprenaline response and lung function was not different in females and males (data not shown); such a difference has been reported for CF adults (49). This might be due to the large number of nonresponders to isoprenaline in our investigations, as expected in a CF cohort of ΔF508 homozygotes representing all ages (32).
Although Cl– conductance presented only in the subgroup of individuals who had better respiratory function, few patients without Cl– conductance possessed relatively good lung function (above the 50th percentile; Figure 4b). This indicates that the capacity to transport Cl– is not the only determinant of respiratory function. Evidently, additional factors unrelated to the basic ion transport defect are involved in CF airway performance. In short, the expression of basal Cl– conductance and the response to isoprenaline in the respiratory tissue were predictive for disease outcome in our cohort of ΔF508 homozygous sibling and twin pairs.
In the intestinal tissue, CFTR-related DIDS-insensitive Cl– secretion was predominantly seen in the mildly affected patients (Figures 6 and 7). Whereas the Ca2+-dependent Cl– secretion in respiratory tissue showed no association with outcome, the DIDS-sensitive Ca2+-dependent Cl– transport pathway was more frequently observed in severely affected ΔF508 homozygotes. These data are supported by a study of the biliary tract in which an inverse relationship was observed between Ca2+-dependent Cl– efflux and cAMP-dependent Cl– transport in homozygous ΔF508 patients (18). The outcome of both studies suggests that alternative Cl– conductance is upregulated in the absence of CFTR activity, but fails to compensate for the lack of cAMP-activated Cl– transport. In contrast, in cftr–/– knockout mice, the upregulation of Ca2+-activated Cl– channel function was associated with healthier tissue function (9, 10).
Although all subjects were homozygous for the same CFTR mutation, CF was found to be heterogeneous even at the level of the basic cellular defect, which should reflect the closest link with the underlying genetic lesion. However, the consistent phenotype of CFTR expression in respiratory and intestinal tracts of a ΔF508 homozygous CF patient (Figure 8) suggests that within 1 person, similar corrective mechanisms lead to maturation, processing, and residual function of ΔF508 CFTR in the major affected organs in CF disease. Furthermore, monozygous twins proved to be significantly more concordant in the electrophysiological properties of their epithelium than dizygous pairs were, especially in the intestinal tract. In addition, their clinical phenotype was even more concordant, since only 1 of the investigated monozygous twin pairs belonged to the DISC group (Table 3 and Table 7). These findings imply that genetic predisposition is important for the expression of Cl– permeability in the respiratory and intestinal tissues (12), and for the clinical phenotype. These influencing genetic factors are located outside the CFTR gene, since all of the investigated sibling pairs were homozygous for the same CFTR lesion.
We cannot formally exclude the possibility that postzygotic events, such as genetic imprinting and/or environmental factors, play an additional role in the differences within sibling pairs. However, siblings share main environmental features such as their physician, therapeutic regimes, living conditions, and microbial contacts.
We conclude that the ability to secrete Cl– in ΔF508 homozygous patients in the organs that are primarily involved in the course of CF disease is predictive of the CF phenotype. Basal Cl– conductance and/or a cAMP-mediated response in the airways, together with DIDS-insensitive residual Cl– secretion in the intestine, were associated with a positive outcome in ΔF508 homozygous CF individuals. Thus, although homozygosity for the major disease-causing genetic lesion was not predictive for disease manifestation and showed a large range of disease severity, the expression of the basic defect was associated with clinical outcome. Clinicians are encouraged not only to use the sweat test, but also to apply NPD and IC measurements to gain more insight into the patient’s electrophysiological characteristics in correlation with disease severity and — possibly in the future — for stratification of clinical trials and treatment of CF disease.
Acknowledgments
The authors cordially thank patients and parents for their cooperation. We are indebted to all members of the European Cystic Fibrosis Twin and Sibling Study Consortium for their continuous support, which was essential in making this study possible. In particular, the organizational cooperation and technical support by the CF teams in Hannover, Innsbruck, London, Rotterdam, and Verona are very much appreciated. The authors thank R. Samlal-Soedhoe for technical assistance in Rotterdam. This work was primarily supported by grants from the Deutsche Forschungsgemeinschaft and the BIOMED II program of the European Union, and in part by the Sophia Foundation for Medical Research (project 235). We gratefully acknowledge the Mukoviszidose eV for financially supporting technical assistance in Hannover (to U. Laabs), and the CF Selbsthilfe eV and the Deutsche Fördergesellschaft für die Mukoviszidoseforschung eV for covering part of the consumables and travel expenses for patients and parents.
References
- 1.Welsh, M.J., Tsui, L.-C., Boat, T.F., and Beaudet, A.L. 1995. Cystic fibrosis. In The metabolic basis of inherited disease. 7th edition. C.R. Scriver, A.L. Beaudet, W.S. Sly, and D. Valle, editors. McGraw-Hill Inc. New York, New York, USA. 3799–3876.
- 2.Anderson MP, Rich DP, Gregory RJ, Smith AE, Welsh MJ. Generation of cAMP-activated chloride currents by expression of CFTR. Science. 1991;251:679–682. doi: 10.1126/science.1704151. [DOI] [PubMed] [Google Scholar]
- 3.Bear CE, et al. Purification and functional reconstitution of the cystic fibrosis transmembrane conductance regulator (CFTR) Cell. 1992;68:809–818. doi: 10.1016/0092-8674(92)90155-6. [DOI] [PubMed] [Google Scholar]
- 4.Kerem B, et al. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245:1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- 5.The Cystic Fibrosis Genetic Analysis Consortium. Population variation of common cystic fibrosis mutations. Hum Mutat. 1994;4:167–177. doi: 10.1002/humu.1380040302. [DOI] [PubMed] [Google Scholar]
- 6.Welsh MJ, Smith AE. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell. 1993;73:1251–1254. doi: 10.1016/0092-8674(93)90353-r. [DOI] [PubMed] [Google Scholar]
- 7.Cheng SH, et al. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell. 1990;63:827–834. doi: 10.1016/0092-8674(90)90148-8. [DOI] [PubMed] [Google Scholar]
- 8.Kartner N, Augustinas O, Jensen TJ, Naismith AL, Riordan JR. Mislocalization of deltaF508 CFTR in cystic fibrosis sweat gland. Nat Genet. 1992;1:321–327. doi: 10.1038/ng0892-321. [DOI] [PubMed] [Google Scholar]
- 9.Clarke LL, et al. Relationship of a non-cystic fibrosis transmembrane conductance regulator-mediated chloride conductance to organ-level disease in Cftr(–/–) mice. Proc Natl Acad Sci USA. 1994;91:479–483. doi: 10.1073/pnas.91.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rozmahel R, et al. Modulation of disease severity in cystic fibrosis transmembrane conductance regulator deficient mice by a secondary genetic factor. Nat Genet. 1996;12:280–287. doi: 10.1038/ng0396-280. [DOI] [PubMed] [Google Scholar]
- 11.Veeze HJ, et al. Determinants of mild symptoms in cystic fibrosis patients: residual chloride secretion measured in rectal biopsies in relation to the genotype. J Clin Invest. 1994;93:461–466. doi: 10.1172/JCI116993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bronsveld I, et al. Residual chloride secretion in intestinal tissue of ΔF508 homozygous twins and siblings with cystic fibrosis. Gastroenterology. 2000;119:32–40. doi: 10.1053/gast.2000.8524. [DOI] [PubMed] [Google Scholar]
- 13.Ho LP, et al. Correlation between nasal potential difference measurements, genotype and clinical condition in patients with cystic fibrosis. Eur Respir J. 1997;10:2018–2022. doi: 10.1183/09031936.97.10092018. [DOI] [PubMed] [Google Scholar]
- 14.Gruber AD, et al. Genomic cloning, molecular characterization, and functional analysis of human CLCA1, the first human member of the family of Ca2+-activated Cl– channel proteins. Genomics. 1998;54:200–214. doi: 10.1006/geno.1998.5562. [DOI] [PubMed] [Google Scholar]
- 15.Gruber AD, Schreur KD, Ji H-L, Fuller CM, Pauli BU. Molecular cloning and transmembrane structure of hCLCA2 from human lung, trachea, and mammary gland. Am J Physiol. 1999;276:C1261–C1270. doi: 10.1152/ajpcell.1999.276.6.C1261. [DOI] [PubMed] [Google Scholar]
- 16.Anderson MP, Sheppard DN, Berger HA, Welsh MJ. Chloride channels in the apical membrane of normal and cystic fibrosis airway and intestinal epithelia. Am J Physiol. 1993;263:L1–L14. doi: 10.1152/ajplung.1992.263.1.L1. [DOI] [PubMed] [Google Scholar]
- 17.Kälin N, Claass A, Sommer M, Puchelle E, Tümmler B. ΔF508 CFTR protein expression in tissues from patients with cystic fibrosis. J Clin Invest. 1999;103:1379–1389. doi: 10.1172/JCI5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dray-Charier N, et al. Expression of delta F508 cystic fibrosis transmembrane conductance regulator protein and related chloride transport properties in the gallbladder epithelium from cystic fibrosis patients. Hepatology. 1999;29:1624–1634. doi: 10.1002/hep.510290634. [DOI] [PubMed] [Google Scholar]
- 19.Risch N, Zhang H. Extreme discordant sib pairs for mapping quantitative trait loci in humans. Science. 1995;268:1584–1589. doi: 10.1126/science.7777857. [DOI] [PubMed] [Google Scholar]
- 20.Risch NJ, Zhang H. Mapping quantitative trait loci with extreme discordant sib pairs: sampling considerations. Am J Hum Genet. 1996;58:836–843. [PMC free article] [PubMed] [Google Scholar]
- 21.Eaves L, Meyer J. Locating human quantitative trait loci: guidelines for the selection of sibling pairs for genotyping. Behav Genet. 1994;24:443–455. doi: 10.1007/BF01076180. [DOI] [PubMed] [Google Scholar]
- 22.Dolan CV, Boomsma DI. Optimal selection of sib pairs from random samples for linkage analysis of a QTL using the EDAC test. Behav Genet. 1998;28:197–206. doi: 10.1023/a:1021423214032. [DOI] [PubMed] [Google Scholar]
- 23.Phillips DI. Twin studies in medical research: can they tell us whether diseases are genetically determined? Lancet. 1993;341:1008–1009. doi: 10.1016/0140-6736(93)91086-2. [DOI] [PubMed] [Google Scholar]
- 24.Sacchetti L, et al. Efficiency of two different nine-loci short tandem repeat systems for DNA typing purposes. Clin Chem. 1999;45:178–183. [PubMed] [Google Scholar]
- 25.Epplen JT, et al. On the potential of simple repetitive DNA for fingerprinting in clinical, forensic, and evolutionary dynamic studies. Clin Investig. 1992;70:1043–1051. doi: 10.1007/BF00180316. [DOI] [PubMed] [Google Scholar]
- 26.Rommens J, et al. Rapid non-radioactive detection of the major cystic fibrosis mutation. Am J Hum Genet. 1990;46:395–396. [PMC free article] [PubMed] [Google Scholar]
- 27.Mekus F, et al. Categories of ΔF508 cystic fibrosis twin and sibling pairs with distinct phenotypic characteristics. Twin Res. 2000;3:277–293. doi: 10.1375/136905200320565256. [DOI] [PubMed] [Google Scholar]
- 28.Prader A, Largo RH, Molinari L, Issler C. Physical growth of Swiss children from birth to 20 years of age. First Zurich longitudinal study of growth and development. Helv Paediatr Acta. 1989;52(Suppl.):1–125. [PubMed] [Google Scholar]
- 29.Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127:725–734. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- 30.Corey M, Edwards L, Levinson H, Knowles M. Longitudinal analysis of pulmonary function decline in patients with cystic fibrosis. J Pediatr. 1997;131:809–814. doi: 10.1016/s0022-3476(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 31.Navarro J, et al. Factors associated with poor pulmonary function: cross-sectional analysis of data from the ERCF. European Epidemiologic Registry of Cystic Fibrosis. Roche Report. Eur Respir J. 2001;18:298–305. doi: 10.1183/09031936.01.00068901. [DOI] [PubMed] [Google Scholar]
- 32.Knowles MR, Paradiso AM, Boucher RC. In vivo nasal potential difference: techniques and protocols for assessing efficacy of gene transfer in cystic fibrosis. Hum Gene Ther. 1995;6:447–457. doi: 10.1089/hum.1995.6.4-445. [DOI] [PubMed] [Google Scholar]
- 33.Canessa CM, et al. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- 34.Mason SJ, Paradiso AM, Boucher RC. Regulation of transepithelial ion transport and intracellular calcium by extracellular ATP in human normal and cystic fibrosis airway epithelium. Br J Pharmacol. 1991;103:1649–1656. doi: 10.1111/j.1476-5381.1991.tb09842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veeze HJ, Sinaasappel M, Bijman J, Bouquet J, de Jonge HR. Ion transport abnormalities in rectal suction biopsies from children with cystic fibrosis. Gastroenterology. 1991;101:398–403. doi: 10.1016/0016-5085(91)90017-f. [DOI] [PubMed] [Google Scholar]
- 36.Calderaro V, et al. Arachidonic acid metabolites and chloride secretion in rabbit distal colonic mucosa. Am J Physiol. 1991;261:G443–G450. doi: 10.1152/ajpgi.1991.261.3.G443. [DOI] [PubMed] [Google Scholar]
- 37.Dharmsathaphorn K, Pandol SJ. Mechanism of chloride secretion induced by carbachol in a colonic epithelial cell line. J Clin Invest. 1986;77:348–354. doi: 10.1172/JCI112311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boige N, Amiranoff B, Munck A, Laburthe M. Forskolin stimulates adenylate cyclase in human colonic crypts: interaction with VIP. Eur J Pharmacol. 1984;101:111–117. doi: 10.1016/0014-2999(84)90036-0. [DOI] [PubMed] [Google Scholar]
- 39.Schwiebert EM, Flotte T, Cutting GR, Guggino WB. Both CFTR and outwardly rectifying chloride channels contribute to cAMP-stimulated whole cell chloride currents. Am J Physiol. 1994;266:C1464–C1477. doi: 10.1152/ajpcell.1994.266.5.C1464. [DOI] [PubMed] [Google Scholar]
- 40.Hardcastle J, Hardcastle PT. The secretory actions of histamine in rat small intestine. J Physiol. 1987;388:521–532. doi: 10.1113/jphysiol.1987.sp016629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dupuit F, et al. CFTR and differentiation markers expression in non-CF and delta F508 homozygous CF nasal epithelium. J Clin Invest. 1995;96:1601–1611. doi: 10.1172/JCI118199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei X, et al. Turnover of the cystic fibrosis transmembrane conductance regulator (CFTR): slow degradation of wild-type and delta F508 CFTR in surface membrane preparations of immortalized airway epithelial cells. J Cell Physiol. 1996;168:373–384. doi: 10.1002/(SICI)1097-4652(199608)168:2<373::AID-JCP16>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 43.Drumm ML, et al. Chloride conductance expressed by delta F508 and other mutant CFTRs in Xenopus oocytes. Science. 1991;254:1797–1799. doi: 10.1126/science.1722350. [DOI] [PubMed] [Google Scholar]
- 44.van Doorninck JH, et al. A mouse model for the cystic fibrosis ΔF508 mutation. EMBO J. 1995;14:4403–4411. doi: 10.1002/j.1460-2075.1995.tb00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelley TJ, Thomas K, Milgram LJH, Drumm ML. In vivo activation of the cystic fibrosis transmembrane conductance regulator mutant ΔF508 in murine nasal epithelium. Proc Natl Acad Sci USA. 1997;94:2604–2608. doi: 10.1073/pnas.94.6.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knowles M, Gatzy J, Boucher R. Increased bioelectric potential difference across respiratory epithelia in cystic fibrosis. N Engl J Med. 1981;305:1489–1495. doi: 10.1056/NEJM198112173052502. [DOI] [PubMed] [Google Scholar]
- 47.Knowles MR, et al. A pilot study of aerosolized amiloride for the treatment of lung disease in cystic fibrosis. N Engl J Med. 1990;322:1189–1194. doi: 10.1056/NEJM199004263221704. [DOI] [PubMed] [Google Scholar]
- 48.Hofmann T, et al. Aerosolized amiloride: dose effect on nasal bioelectric properties, pharmacokinetics, and effect on sputum expectoration in patients with cystic fibrosis. J Aerosol Med. 1997;10:147–158. doi: 10.1089/jam.1997.10.147. [DOI] [PubMed] [Google Scholar]
- 49.Thomas SR, Jaffe A, Geddes DM, Hodson ME, Alton EW. Pulmonary disease severity in men with ΔF508 cystic fibrosis and residual chloride secretion. Lancet. 1999;353:984–985. doi: 10.1016/S0140-6736(98)05447-6. [DOI] [PubMed] [Google Scholar]
- 50.Bijman, J., et al. 1988. Electrolyte transport in normal and CF epithelia. In Exocrine secretion. J.A. Young and P.Y. Wong, editors. Hong Kong University Press. Hong Kong. 17–19.