Abstract
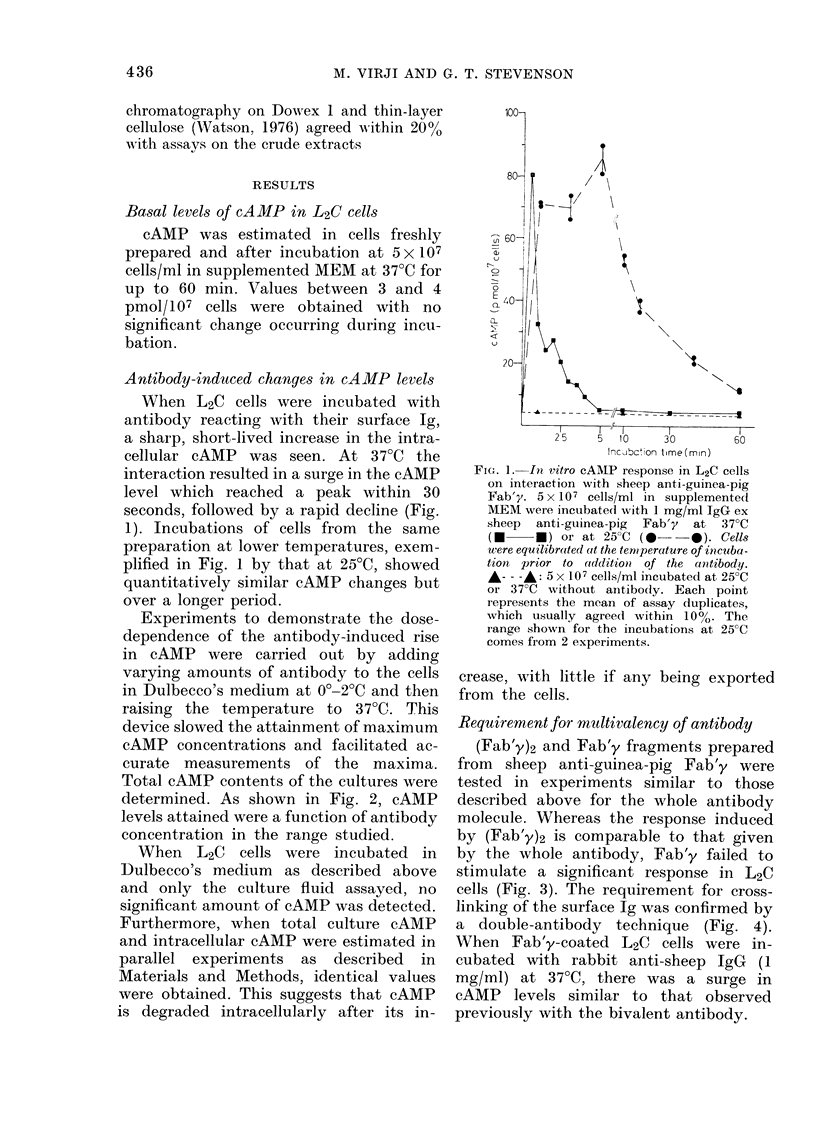
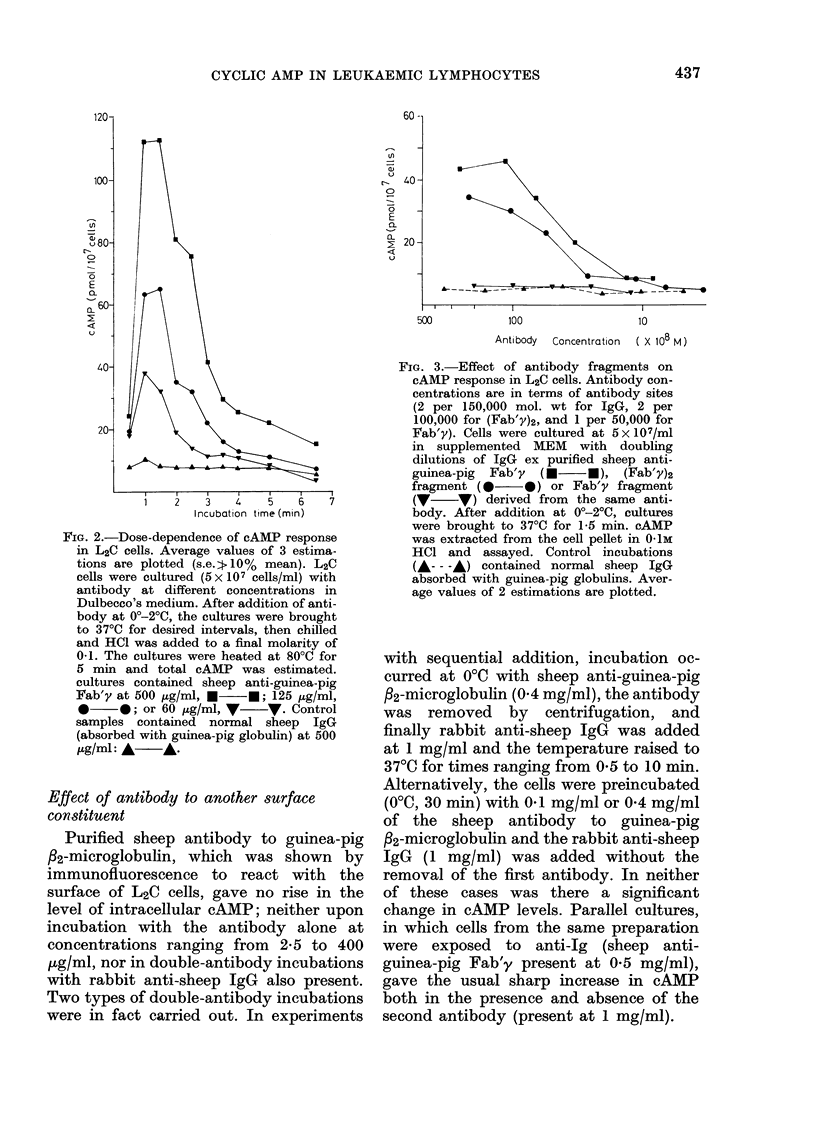
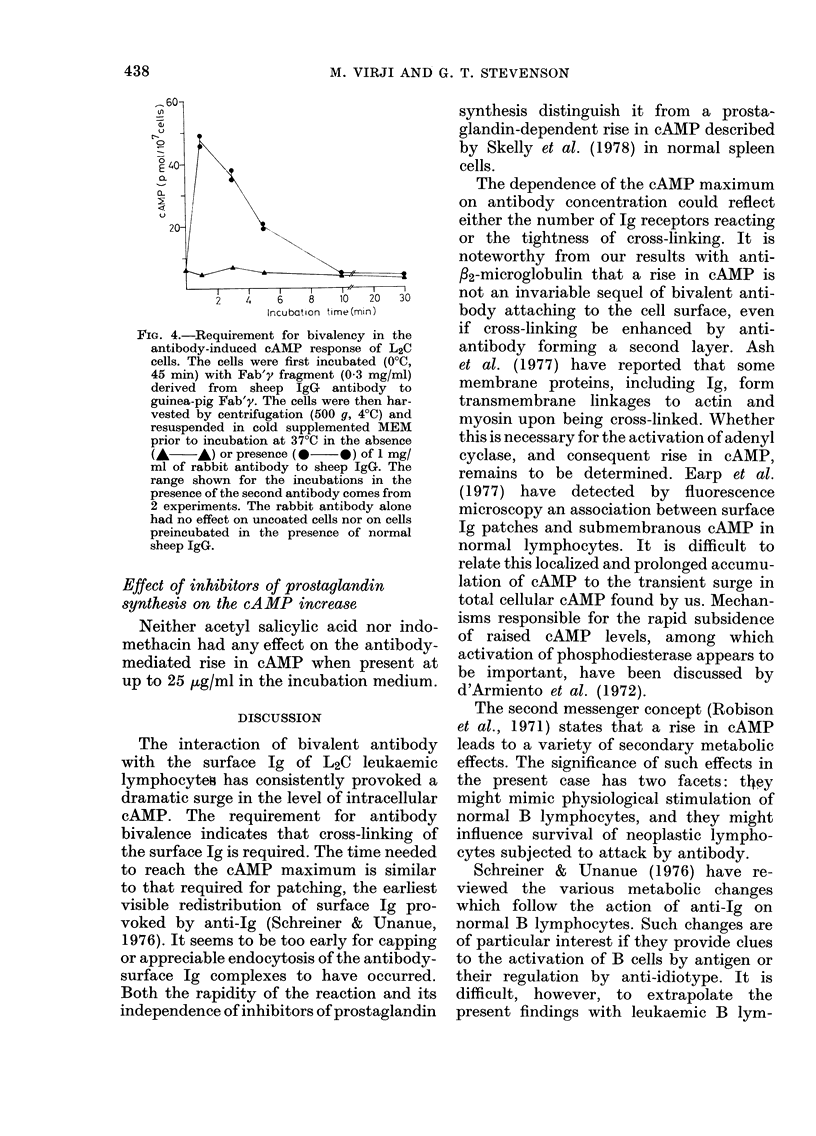
When L2C leukaemic B lymphocytes from guinea-pigs were incubated in vitro with antibody directed to their surface immunoglobulin (Ig), a rapid rise in intracellular adenosine 3':5'-phosphate (cyclic adenosine monophosphate, cAMP) was observed. Estimation of cAMP was by a protein-binding assay using bovine adrenal protein kinase. Increases up to 30-fold occurred within 30 seconds of incubation at 37 degrees C, to be succeeded by a fall which reached the basal level between 5 and 7 min. The response was proportional to the amount of antibody present. Cross-linking of surface Ig by the antibody was necessary, bivalent (Fab'gamma)2 from the antibody gave a rise in cAMP similar to that given by the parent molecule, whereas monomeric Fab'gamma was ineffective unless it was subsequently cross-linked by anti-antibody. The rise was too rapid to have required capping of the surface Ig for its induction. Not all perturbations of the plasma membrane by antibody induce such a surge in cAMP, since anti-beta2 microglobulin, also reacting with the lymphocyte surface, failed to alter cAMP concentration. The results emphasize that immunotherapy can be influenced by antibody altering the metabolic activity of target cells, quite apart from activation of immunological cytotoxic pathways.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ash J. F., Louvard D., Singer S. J. Antibody-induced linkages of plasma membrane proteins to intracellular actomyosin-containing filaments in cultured fibroblasts. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5584–5588. doi: 10.1073/pnas.74.12.5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Brown B. L., Albano J. D., Ekins R. P., Sgherzi A. M. A simple and sensitive saturation assay method for the measurement of adenosine 3':5'-cyclic monophosphate. Biochem J. 1971 Feb;121(3):561–562. doi: 10.1042/bj1210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran A. J., Kiessling R., Klein E., Gunvén P., Foulis A. K. Human tumor cell migration. J Natl Cancer Inst. 1973 Oct;51(4):1109–1111. doi: 10.1093/jnci/51.4.1109. [DOI] [PubMed] [Google Scholar]
- D'Armiento M., Johnson G. S., Pastan I. Regulation of adenosine 3',5'-cyclic monophosphate phosphodiesterase activity in fibroblasts by intracellular concentrations of cyclic adenosine monophosphate (3T3-dibutyryl cyclic AMP-SV40-transformed cells-michaelis constants-L cells-prostaglandin E 1 ). Proc Natl Acad Sci U S A. 1972 Feb;69(2):459–462. doi: 10.1073/pnas.69.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earp H. S., Utsinger P. D., Yount W. J., Logue M., Steiner A. L. Lymphocyte surface modulation and cyclic nucleotides I. Topographic correlation of cyclic adenosine 3':5'-monophosphate and immunoglobulin immunofluorescence during lymphocyte capping. J Exp Med. 1977 Apr 1;145(4):1087–1092. doi: 10.1084/jem.145.4.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough D. W., Chapple J. C., Stevenson F. K., Stevenson G. T. Further studies of immunoglobulin synthesis by guinea pig leukaemic lymphocytes. Immunology. 1978 May;34(5):889–899. [PMC free article] [PubMed] [Google Scholar]
- Hough D. W., Eady R. P., Hamblin T. J., Stevenson F. K., Stevenson G. T. Anti-idiotype sera raised against surface immunoglobulin of human neoplastic lymphocytes. J Exp Med. 1976 Oct 1;144(4):960–969. doi: 10.1084/jem.144.4.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. J., Pasternack G. R., Shin H. S. Antibody-mediated suppression of tumor growth. I. Suppression by murine IgG1 isolated from alloantiserum. J Immunol. 1977 Feb;118(2):489–493. [PubMed] [Google Scholar]
- Motta R. Passive immunotherapy of leukemia and other cancer. Adv Cancer Res. 1971;14:161–179. doi: 10.1016/s0065-230x(08)60520-5. [DOI] [PubMed] [Google Scholar]
- Nishizawa Y., Kishimoto T., Kikutani H., Yamamura Y. Induction and properties of cytoplasmic factor(s) which enhance nuclear nonhistone protein phosphorylation in lymphocytes stimulated by anti-Ig. J Exp Med. 1977 Sep 1;146(3):653–664. doi: 10.1084/jem.146.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill G. J. Control of an EL4 lymphoma in nude mice by passively administered antibody. Eur J Cancer. 1976 Sep;12(9):749–751. doi: 10.1016/0014-2964(76)90027-x. [DOI] [PubMed] [Google Scholar]
- ROSS A., LEPOW I. H. Studies on immune cellular injury. I. Cytotoxic effects of antibody and complement. J Exp Med. 1960 Dec 1;112:1085–1106. doi: 10.1084/jem.112.6.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S. A., Terry W. D. Passive immunotherapy of cancer in animals and man. Adv Cancer Res. 1977;25:323–388. doi: 10.1016/s0065-230x(08)60637-5. [DOI] [PubMed] [Google Scholar]
- Rubens R. D., Vaughan-Smith S., Dulbecco R. Augmentation of cytotoxic drug action and X-irradiation by antibodies. Br J Cancer. 1975 Sep;32(3):352–354. doi: 10.1038/bjc.1975.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner G. F., Unanue E. R. Membrane and cytoplasmic changes in B lymphocytes induced by ligand-surface immunoglobulin interaction. Adv Immunol. 1976;24:37–165. doi: 10.1016/s0065-2776(08)60329-6. [DOI] [PubMed] [Google Scholar]
- Shearer W. T., Atkinson J. P., Parker C. W. Humoral immunostimulation. VI. Increased calcium uptake by cells treated with antibody and complement. J Immunol. 1976 Sep;117(3):973–980. [PubMed] [Google Scholar]
- Shearer W. T., Parker C. W. Antibody and complement modulation of tumor cell growth in vitro and in vivo. Fed Proc. 1978 Aug;37(10):2385–2389. [PubMed] [Google Scholar]
- Skelly R. R., Steinberg A. D., Plescia O. J. Regulation of antigen induced changes in cyclic nucleotide levels in NZB/wf1 mice. Cell Immunol. 1978 Mar 15;36(2):283–293. doi: 10.1016/0008-8749(78)90272-1. [DOI] [PubMed] [Google Scholar]
- Stevenson F. K., Cleeter M. W., Stevenson G. T. beta2-Microglobulin from normal and leukaemic guinea-pig lymphocytes. Scand J Immunol. 1978;8(2):127–134. doi: 10.1111/j.1365-3083.1978.tb00504.x. [DOI] [PubMed] [Google Scholar]
- Stevenson G. T., Eady R. P., Hough D. W., Jurd R. D., Stevenson F. K. Surface immunoglobulin of guinea-pig leukaemic lymphocytes. Immunology. 1975 May;28(5):807–820. [PMC free article] [PubMed] [Google Scholar]
- Stevenson G. T., Elliott E. V., Stevenson F. K. Idiotypic determinants on the surface immunoglobulin of neoplastic lymphocytes: a therapeutic target. Fed Proc. 1977 Aug;36(9):2268–2271. [PubMed] [Google Scholar]
- Stevenson G. T., Stevenson F. K. Antibody to a molecularly-defined antigen confined to a tumour cell surface. Nature. 1975 Apr 24;254(5502):714–716. doi: 10.1038/254714a0. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Ault K. A., Karnovsky M. J. Ligand-induced movement of lymphocyte surface macromolecules. IV. Stimulation of cell motility by anti-Ig and lack of relationship to capping. J Exp Med. 1974 Feb 1;139(2):295–312. doi: 10.1084/jem.139.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. The involvement of cyclic nucleotide metabolism in the initiation of lymphocyte proliferation induced by mitogens. J Immunol. 1976 Nov;117(5 Pt 1):1656–1663. [PubMed] [Google Scholar]
- Yutoku M., Grossberg A. L., Pressman D. Suppression of in vivo growth of mouse myelomas by purified rabbit antibodies against mouse myeloma cells. J Natl Cancer Inst. 1974 Jul;53(1):201–207. doi: 10.1093/jnci/53.1.201. [DOI] [PubMed] [Google Scholar]
- Zighelboim J., Bonavida B., Fahey J. L. Antibody-mediated in vivo suppression of EL4 leukemia in a syngeneic host. J Natl Cancer Inst. 1974 Mar;52(3):879–881. doi: 10.1093/jnci/52.3.879. [DOI] [PubMed] [Google Scholar]


