Abstract
Male Wistar rats fed a normal laboratory pelleted diet, when treated s.c. with 1,2-dimethylhydrazine (DMH) 10 mg/kg/wk survived the 24-week experiment, showed no signs of chemical toxicity or macroscopic liver damage, and developed mainly large-bowel tumours. Conversely, male Wistar rats treated with 20 mg/kg/wk DMH did not survive the full term of the experiment and developed ascites, pleural effusions and nodular livers. They also developed more small-bowel tumours than large-bowel tumours. The relationship between the predominant site of tumour development and dosage of DMH was highly significant.
Male Wistar rats fed with an all-liquid diet (Vivonex) and treated with 20 mg/kg/wk DMH behaved quite differently both in terms of survival and site of tumour development. These rats survived the full term of the experiment, showed no signs of chemical toxicity, experienced minimal liver damage and developed predominantly large-bowel tumours. The protection afforded by the all-liquid diet against DMH toxicity and small-bowel tumour induction was statistically highly significant.
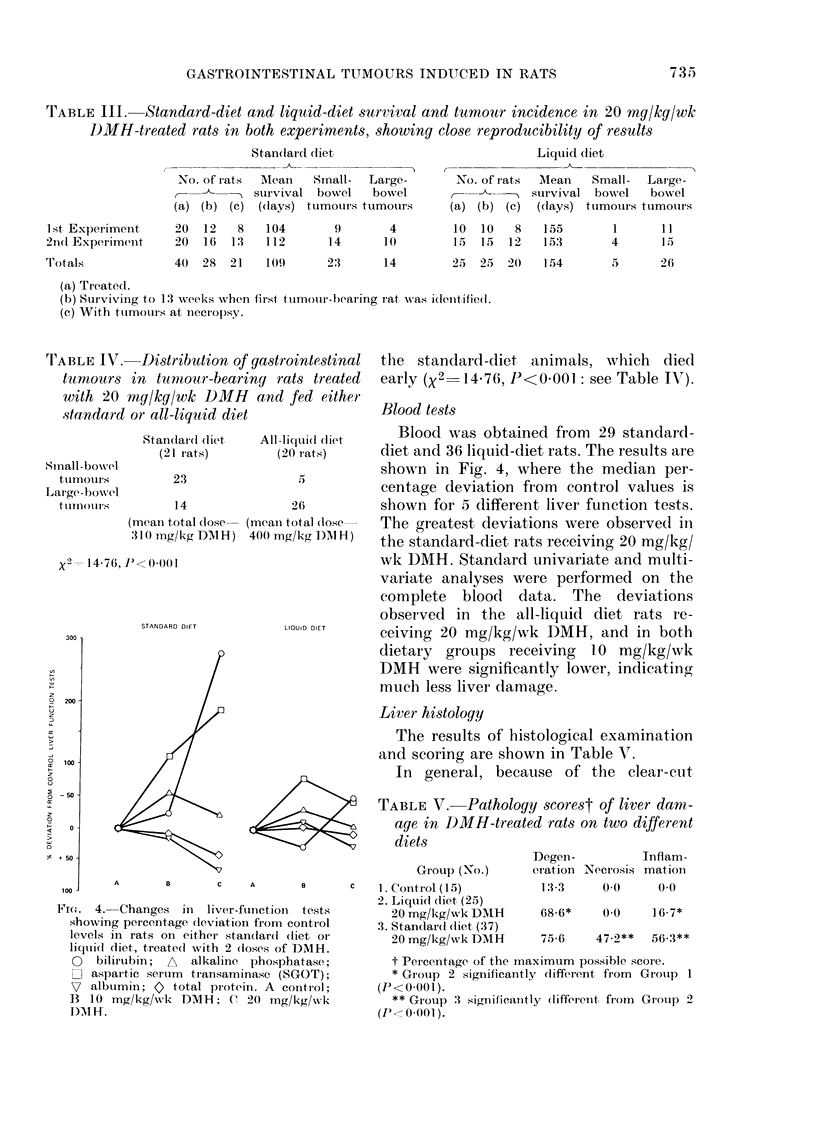
A series of blood tests with special reference to liver function confirmed the highly significant degree of protection against liver damage afforded by the all-liquid diet.
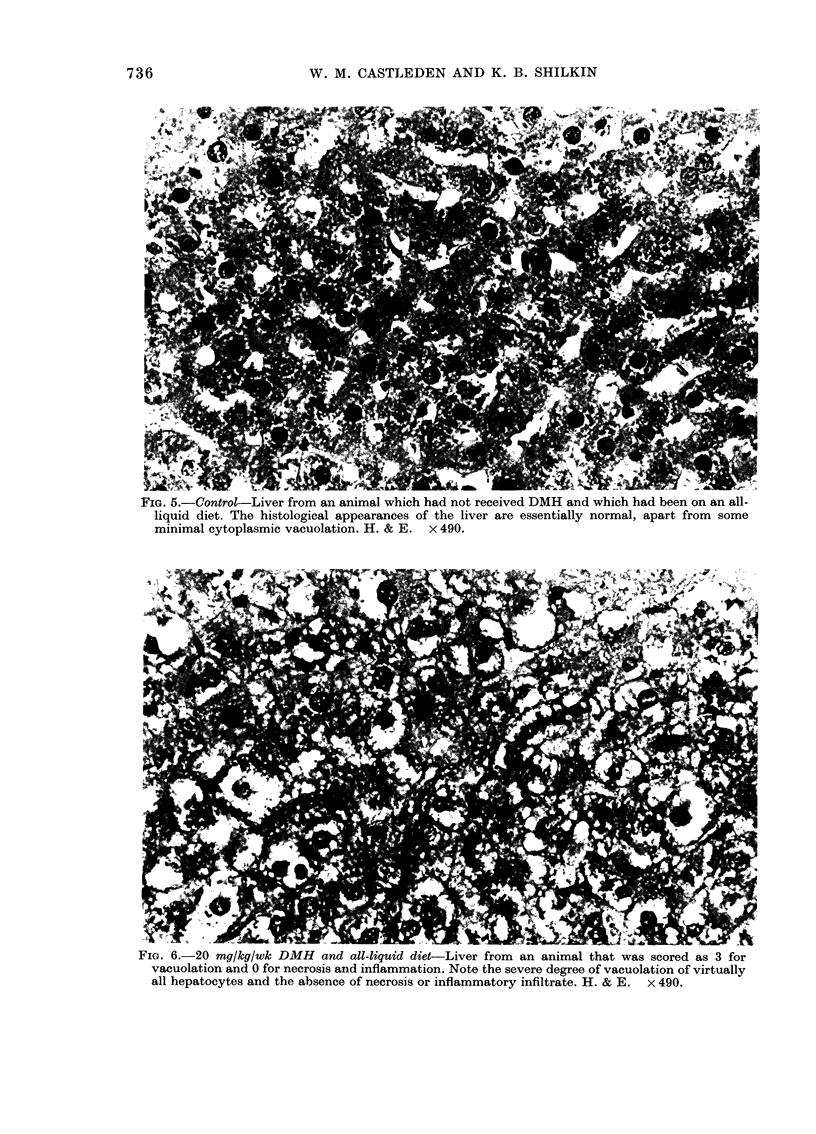
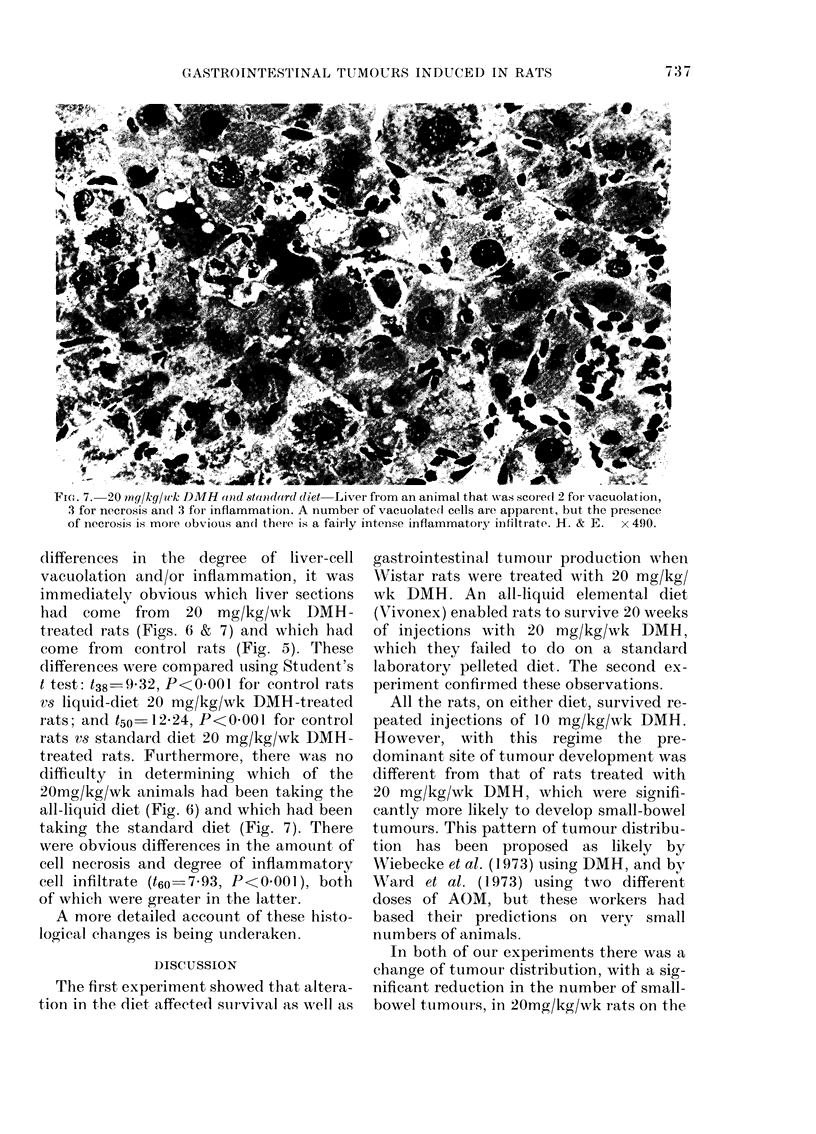
Sections of liver from treated rats were examined, and a simple pathological scoring system was devised which showed a highly significant difference in liver histology between standard diet and liquid-diet rats treated with 20 mg/kg/wk DMH.
The results strongly suggest an association between severity of liver damage from DMH and the subsequent development of small-bowel tumours. The all-liquid diet protected rats from liver damage and these rats developed significantly fewer small-bowel tumours.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burkitt D. P. Some diseases characteristic of modern Western civilization. Br Med J. 1973 Feb 3;1(5848):274–278. doi: 10.1136/bmj.1.5848.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castleden W. M. Prolonged survival and decrease in intestinal tumours in dimethylhydrazine-treated rats fed a chemically defined diet. Br J Cancer. 1977 Apr;35(4):491–495. doi: 10.1038/bjc.1977.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala E. S. Investigations into the metabolism and mode of action of the colon carcinogens 1,2-dimethylhydrazine and azoxymethane. Cancer. 1977 Nov;40(5 Suppl):2436–2445. doi: 10.1002/1097-0142(197711)40:5+<2436::aid-cncr2820400908>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Fiala E. Investigations into the metabolism and mode of action of the colon carcinogen 1, 2-dimethylhydrazine. Cancer. 1975 Dec;36(6 Suppl):2407–2412. doi: 10.1002/1097-0142(197512)36:6<2407::aid-cncr2820360620>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Hawks A., Hicks R. M., Holsman J. W., Magee P. N. Morphological and biochemical effects of 1,2-dimethylhydrazine and 1-methylhydrazine in rats and mice. Br J Cancer. 1974 Nov;30(5):429–439. doi: 10.1038/bjc.1974.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawks A., Magee P. N. The alkylation of nucleic acids of rat and mouse in vivo by the carcinogen 1,2-dimethylhydrazine. Br J Cancer. 1974 Nov;30(5):440–447. doi: 10.1038/bjc.1974.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger C. Chemical carcinogenesis. Cancer. 1977 Jul;40(1 Suppl):430–433. doi: 10.1002/1097-0142(197707)40:1+<430::aid-cncr2820400703>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Hill M. J. Metabolic epidemiology of dietary factors in large bowel cancer. Cancer Res. 1975 Nov;35(11 Pt 2):3398–3402. [PubMed] [Google Scholar]
- Hill M. J., Morson B. C., Bussey H. J. Aetiology of adenoma--carcinoma sequence in large bowel. Lancet. 1978 Feb 4;1(8058):245–247. doi: 10.1016/s0140-6736(78)90487-7. [DOI] [PubMed] [Google Scholar]
- Laqueur G. L., Spatz M. Toxicology of cycasin. Cancer Res. 1968 Nov;28(11):2262–2267. [PubMed] [Google Scholar]
- Pratt C. B., Rivera G., Shanks E., Johnson W. W., Howarth C., Terrell W., Kumar A. P. Colorectal carcinoma in adolescents implications regarding etiology. Cancer. 1977 Nov;40(5 Suppl):2464–2472. doi: 10.1002/1097-0142(197711)40:5+<2464::aid-cncr2820400912>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Shank R. C., Magee P. N. Similarities between the biochemical actions of cycasin and dimethylnitrosamine. Biochem J. 1967 Nov;105(2):521–527. doi: 10.1042/bj1050521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale J. J. Possible role of nutrients in neoplasia. Cancer Res. 1975 Nov;35(11 Pt 2):3320–3325. [PubMed] [Google Scholar]
- Ward J. M., Yamamoto R. S., Brown C. A. Pathology of intestinal neoplasms and other lesions in rats exposed to azoxymethane. J Natl Cancer Inst. 1973 Sep;51(3):1029–1039. doi: 10.1093/jnci/51.3.1029. [DOI] [PubMed] [Google Scholar]
- Weisburger J. H. Colon carcinogens: their metabolism and mode of action. Cancer. 1971 Jul;28(1):60–70. doi: 10.1002/1097-0142(197107)28:1<60::aid-cncr2820280113>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Wiebecke B., Krey U., Löhrs U., Eder M. Morphological and autoradiographical investigations on experimental carcinogenesis and polyp development in the intestinal tract of rats and mice. Virchows Arch A Pathol Pathol Anat. 1973 Sep 4;360(3):179–193. doi: 10.1007/BF00542979. [DOI] [PubMed] [Google Scholar]
- Wynder E. L. The epidemiology of large bowel cancer. Cancer Res. 1975 Nov;35(11 Pt 2):3388–3394. [PubMed] [Google Scholar]





