Abstract
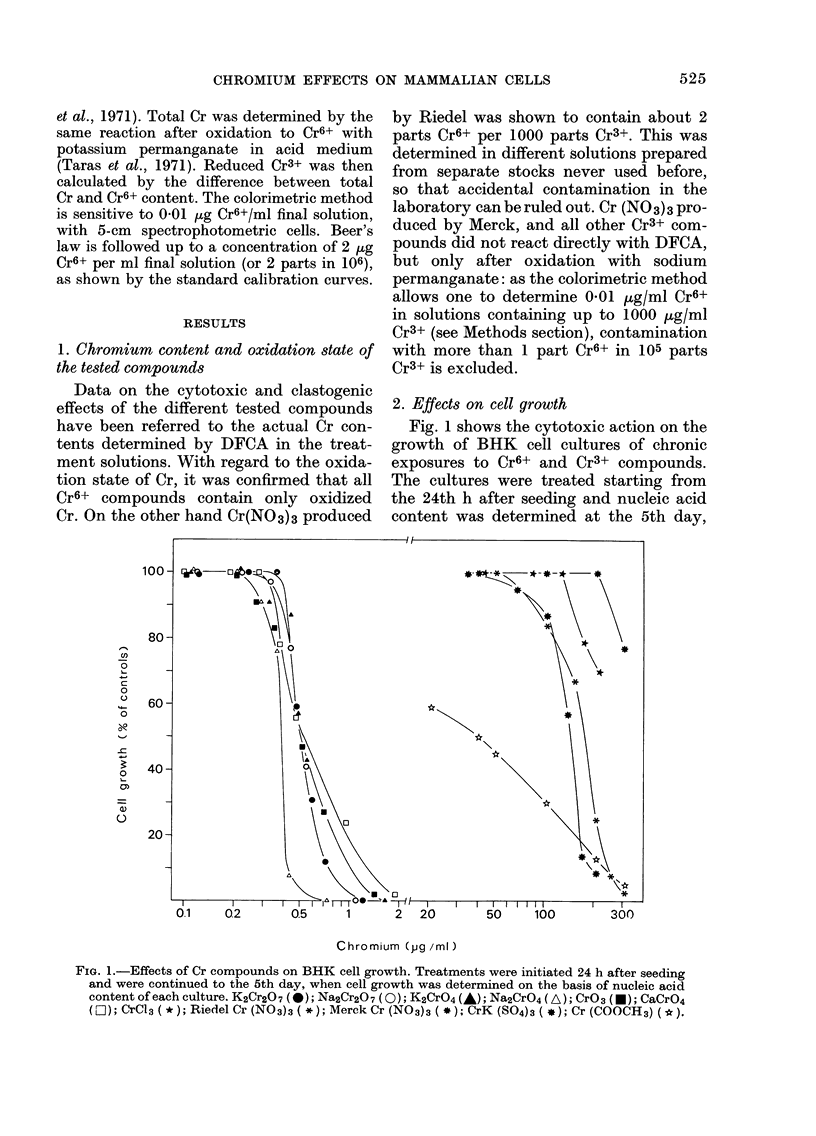
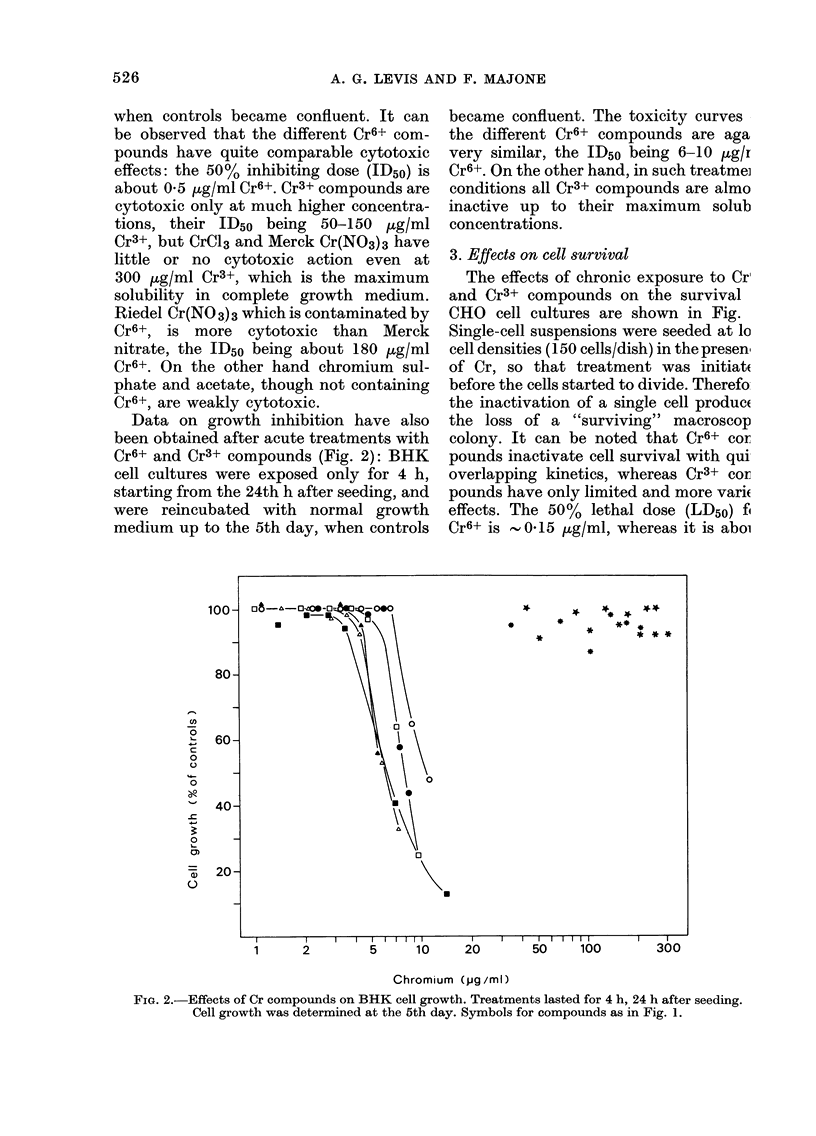
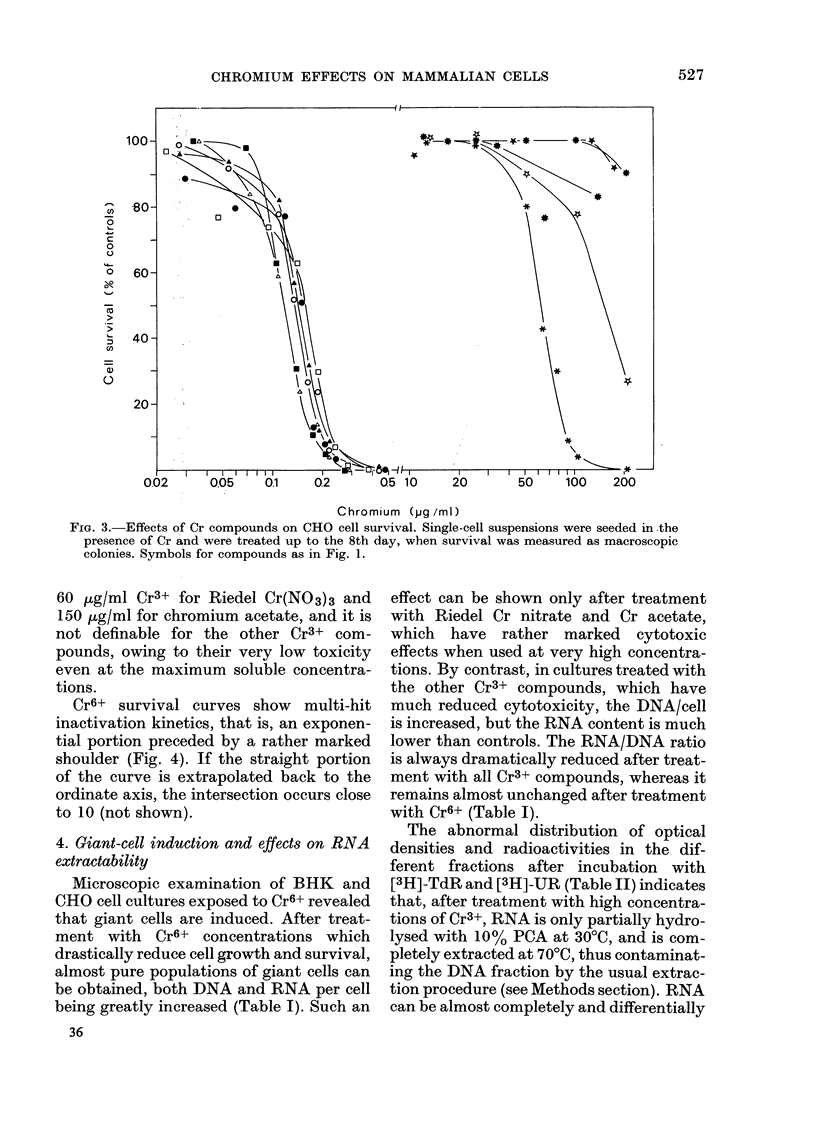
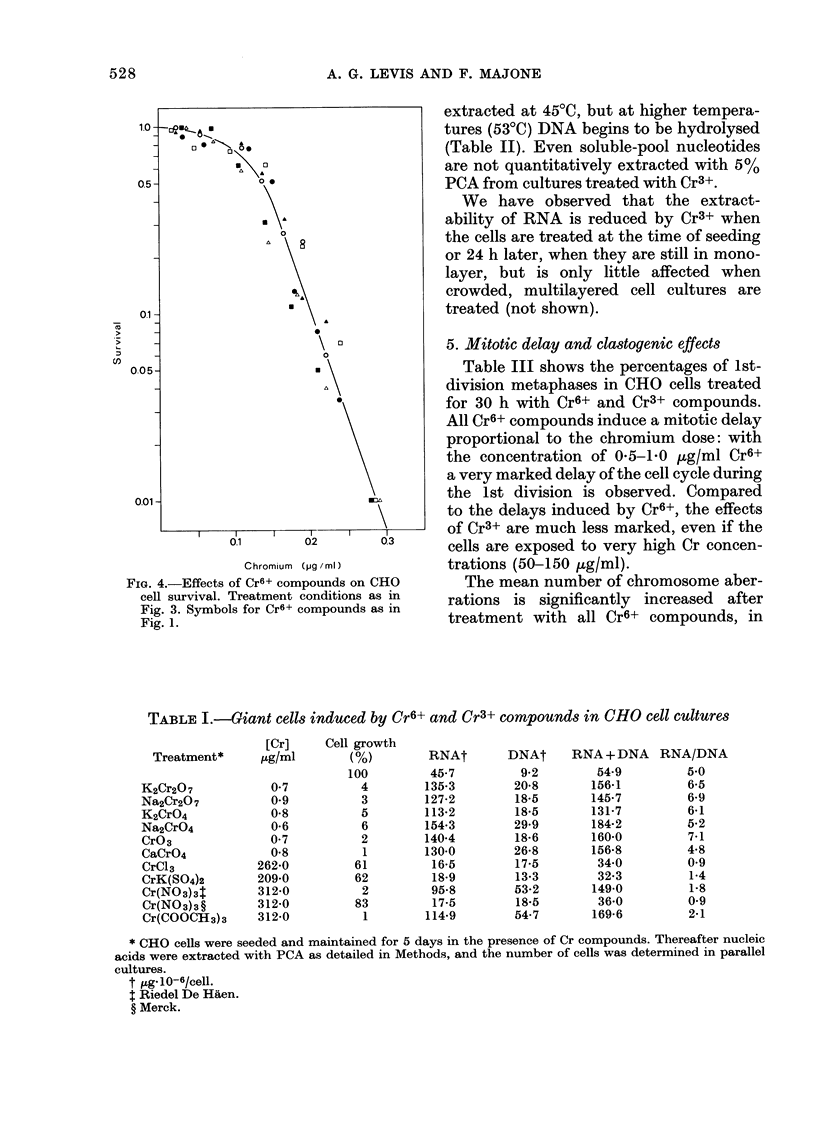
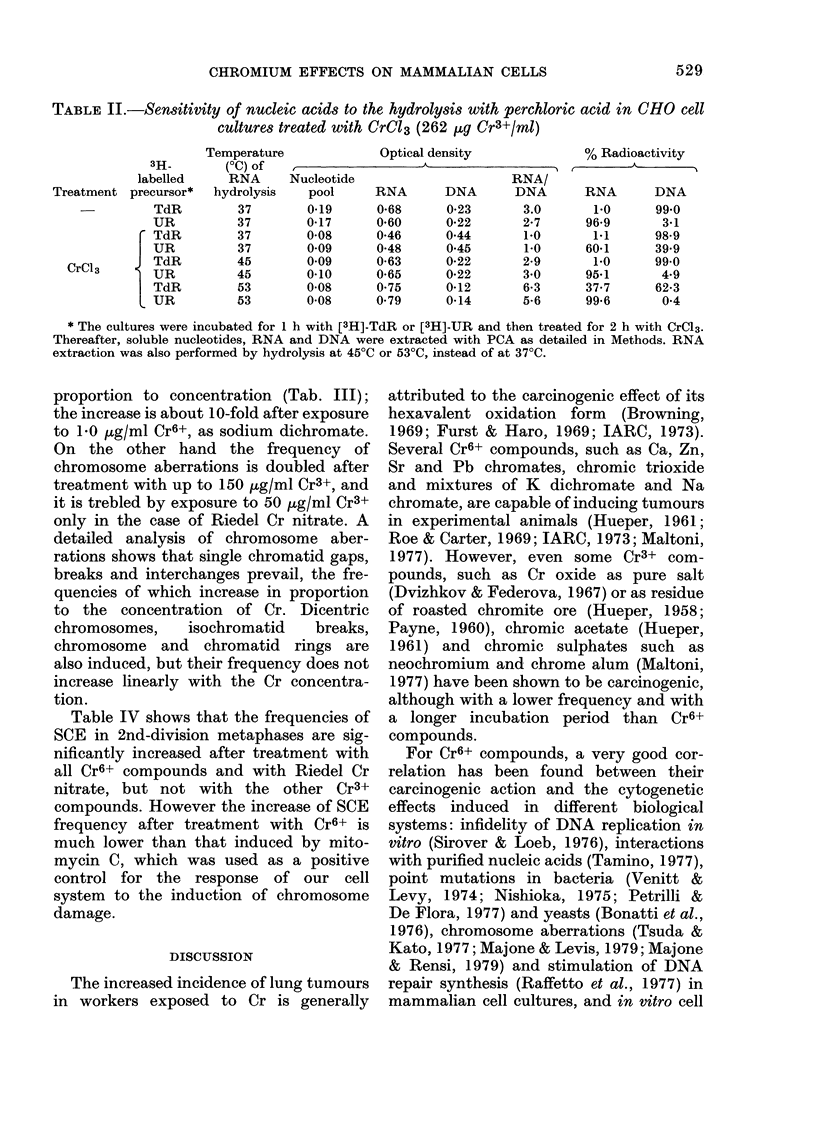
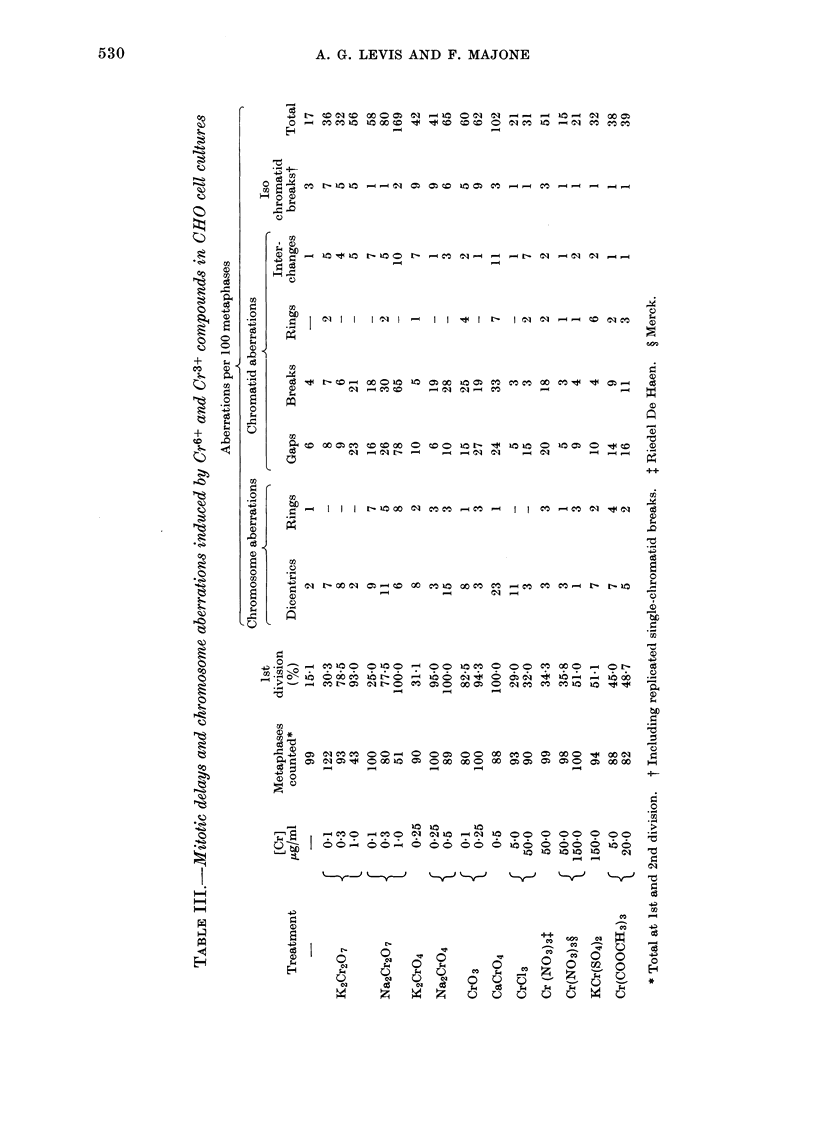
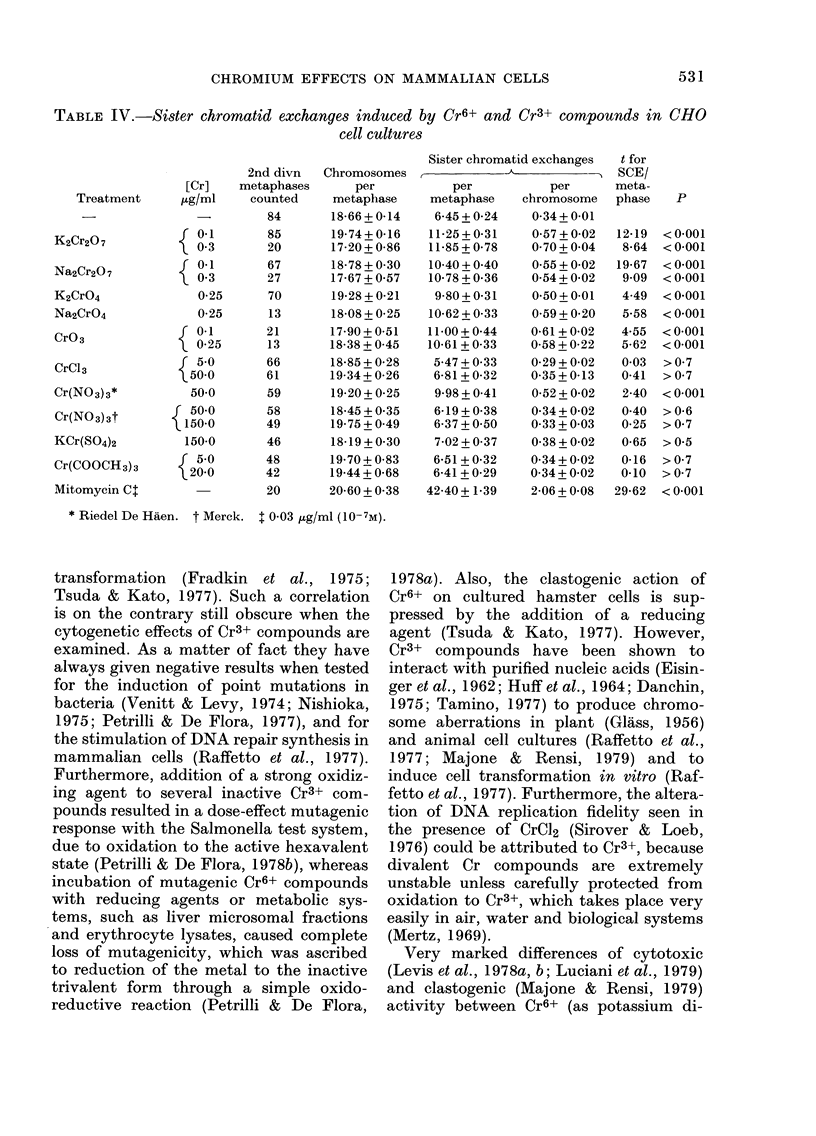
The inhibition of cell growth, the reduction of cell survival and the induction of chromosome aberrations and of sister chromatid exchange (SCE) have been determined in cultured hamster cell lines (BHK and CHO) treated with 11 water-soluble compounds of hexavalent and trivalent chromium. All Cr6+ compounds inhibit growth of BHK cells and reduce survival of CHO cells to levels comparable to those obtained only after exposure to 100--1000 times higher Cr3+ concentrations. The cytotoxicity curves obtained with the different Cr6+ compounds are almost overlapping, whereas marked differences of activity are noticeable among Cr3+ compounds. Giant cells are obtained after exposure to Cr6+ and Cr3+ compounds, as shown by the rise of DNA and RNA per cell, and are due to the blockage of the cell cycle without sudden inhibition of macromolecular syntheses. Both Cr6+ and Cr3+ compounds are able to induce chromosome aberrations, whereas Cr3+ is absolutely incapable of inducing SCE, only Cr6+ being active. The frequency of chromosome aberrations is increased about 10-fold after exposure to 1.0 micrograms/ml Cr6+, whereas it is only doubled after treatment with up to 150 micrograms/ml Cr3+. On the other hand, in spite of the sensitivity of CHO cells to the induction of SCE by mitomycin C, the frequency of SCE hardly doubles after exposure to Cr6+ compounds. The present data confirm that Cr6+ compounds are characterized by a marked cytotoxicity and clastogenic action on mammalian cell cultures and show that Cr3+ compounds, though cytotoxic only at extremely high concentrations and not increasing the frequency of SCE, are not completely without cytogenetic effect, as they are able to induce chromosome aberrations.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bianchi V., Levis A. G., Saggioro D. Differential cytotoxic activity of potassium dichromate on nucleoside uptake in BHK fibroblasts. Chem Biol Interact. 1979 Feb;24(2):137–151. doi: 10.1016/0009-2797(79)90003-6. [DOI] [PubMed] [Google Scholar]
- Bonatti S., Meini M., Abbondandolo A. Genetic effects of potassium dichromate in Schizosaccharomyces pombe. Mutat Res. 1976 Apr;38(2):147–150. [PubMed] [Google Scholar]
- Dvizhkov P. P., Fedorova V. I. O blastomogennykh svoistvakh okisi khroma. Vopr Onkol. 1967;13(11):57–62. [PubMed] [Google Scholar]
- Fradkin A., Janoff A., Lane B. P., Kuschner M. In vitro transformation of BHK21 cells grown in the presence of calcium chromate. Cancer Res. 1975 Apr;35(4):1058–1063. [PubMed] [Google Scholar]
- Furst A., Haro R. T. A survey of metal carcinogenesis. Prog Exp Tumor Res. 1969;12:102–133. [PubMed] [Google Scholar]
- HUEPER W. C. Environmental carcinogenesis and cancers. Cancer Res. 1961 Aug;21:842–857. [PubMed] [Google Scholar]
- HUEPER W. C. Experimental studies in metal cancerigenesis. X. Cancerigenic effects of chromite ore roast deposited in muscle tissue and pleural cavity of rats. AMA Arch Ind Health. 1958 Oct;18(4):284–291. [PubMed] [Google Scholar]
- HUFF J. W., SASTRY K. S., GORDON M. P., WACKER W. E. THE ACTION OF METAL IONS ON TOBACCO MOSAIC VIRUS RIBONUCLEIC ACID. Biochemistry. 1964 Apr;3:501–506. doi: 10.1021/bi00892a006. [DOI] [PubMed] [Google Scholar]
- Korenberg J. R., Freedlender E. F. Giemsa technique for the detection of sister chromatid exchanges. Chromosoma. 1974;48(4):355–360. doi: 10.1007/BF00290992. [DOI] [PubMed] [Google Scholar]
- Levis A. G., Bianchi V., Tamino G., Pegoraro B. Cytotoxic effects of hexavalent and trivalent chromium on mammalian cells in vitro. Br J Cancer. 1978 Mar;37(3):386–396. doi: 10.1038/bjc.1978.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis A. G., Buttignol M., Bianchi V., Sponza G. Effects of potassium dichromate on nucleic acid and protein syntheses and on precursor uptake in BHK fibroblasts. Cancer Res. 1978 Jan;38(1):110–116. [PubMed] [Google Scholar]
- Majone F., Levis A. G. Chromosomal aberrations and sister-chromatid exchanges in Chinese hamster cells treated in vitro with hexavalent chromium compounds. Mutat Res. 1979 Jul;67(3):231–238. doi: 10.1016/0165-1218(79)90017-x. [DOI] [PubMed] [Google Scholar]
- Maltoni C. Occupational carcinogenesis. Predictive value of carcinogenesis bioassays. Ann N Y Acad Sci. 1976;271:431–443. doi: 10.1111/j.1749-6632.1976.tb23144.x. [DOI] [PubMed] [Google Scholar]
- Mertz W. Chromium occurrence and function in biological systems. Physiol Rev. 1969 Apr;49(2):163–239. doi: 10.1152/physrev.1969.49.2.163. [DOI] [PubMed] [Google Scholar]
- Nishioka H. Mutagenic activities of metal compounds in bacteria. Mutat Res. 1975 Jun;31(3):185–189. doi: 10.1016/0165-1161(75)90088-6. [DOI] [PubMed] [Google Scholar]
- PAYNE W. W. The role of roasted chromite ore in the production of cancer. Arch Environ Health. 1960 Jul;1:20–26. doi: 10.1080/00039896.1960.10662663. [DOI] [PubMed] [Google Scholar]
- Petrilli F. L., De Flora S. Metabolic deactivation of hexavalent chromium mutagenicity. Mutat Res. 1978 Oct;54(2):139–147. doi: 10.1016/0165-1161(78)90034-1. [DOI] [PubMed] [Google Scholar]
- Petrilli F. L., De Flora S. Toxicity and mutagenicity of hexavalent chromium on Salmonella typhimurium. Appl Environ Microbiol. 1977 Apr;33(4):805–809. doi: 10.1128/aem.33.4.805-809.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrilli F. L., de Flora S. Oxidation of inactive trivalent chromium to the mutagenic hexavalent form. Mutat Res. 1978 Nov;58(2-3):167–173. doi: 10.1016/0165-1218(78)90006-x. [DOI] [PubMed] [Google Scholar]
- Raffetto G., Parodi S., Parodi C., De Ferrari M., Troiano R., Brambilla G. Direct interaction with cellular targets as the mechanism for chromium carcinogenesis. Tumori. 1977 Nov-Dec;63(6):503–512. doi: 10.1177/030089167706300602. [DOI] [PubMed] [Google Scholar]
- Roe F. J., Carter R. L. Chromium carcinogenesis: calcium chromate as a potent carcinogen for the subcutaneous tissues of the rat. Br J Cancer. 1969 Mar;23(1):172–176. doi: 10.1038/bjc.1969.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirover M. A., Loeb L. A. Infidelity of DNA synthesis in vitro: screening for potential metal mutagens or carcinogens. Science. 1976 Dec 24;194(4272):1434–1436. doi: 10.1126/science.1006310. [DOI] [PubMed] [Google Scholar]
- Tsuda H., Kato K. Chromosomal aberrations and morphological transformation in hamster embryonic cells treated with potassium dichromate in vitro. Mutat Res. 1977 Apr;46(2):87–94. doi: 10.1016/0165-1161(77)90115-7. [DOI] [PubMed] [Google Scholar]
- Venitt S., Levy L. S. Mutagenicity of chromates in bacteria and its relevance to chromate carcinogenesis. Nature. 1974 Aug 9;250(5466):493–495. doi: 10.1038/250493a0. [DOI] [PubMed] [Google Scholar]


