Abstract
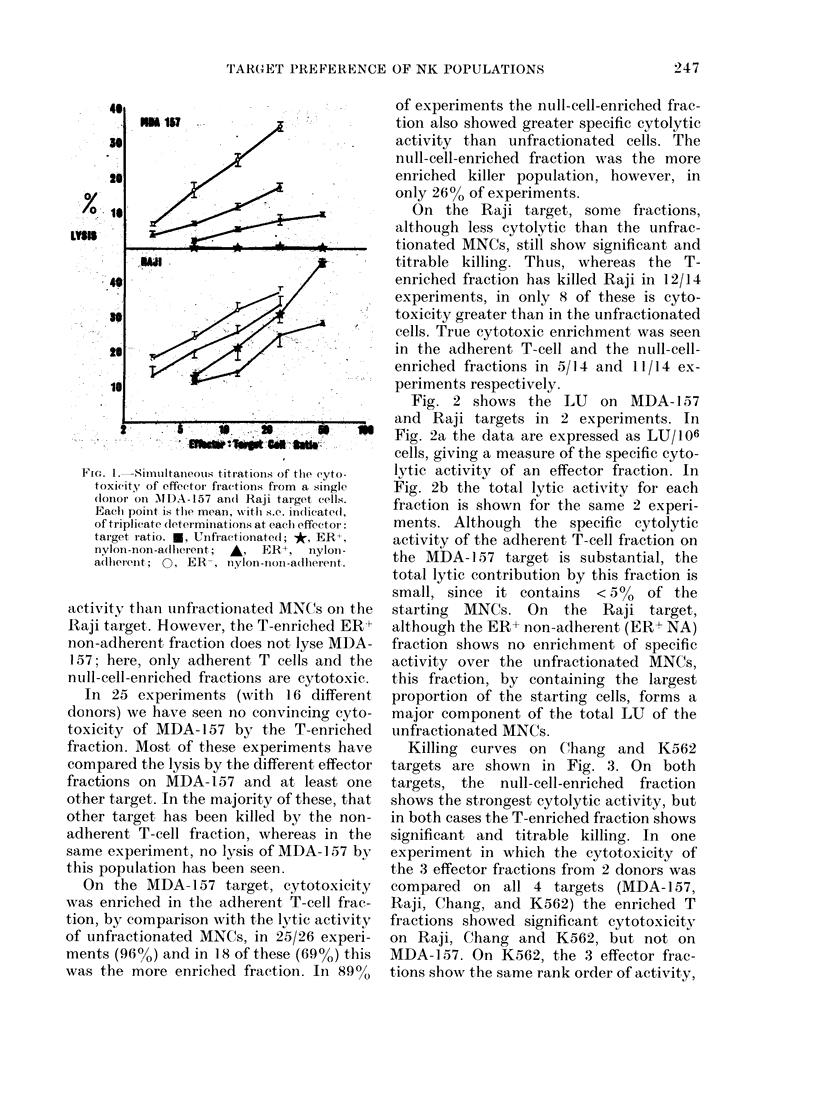
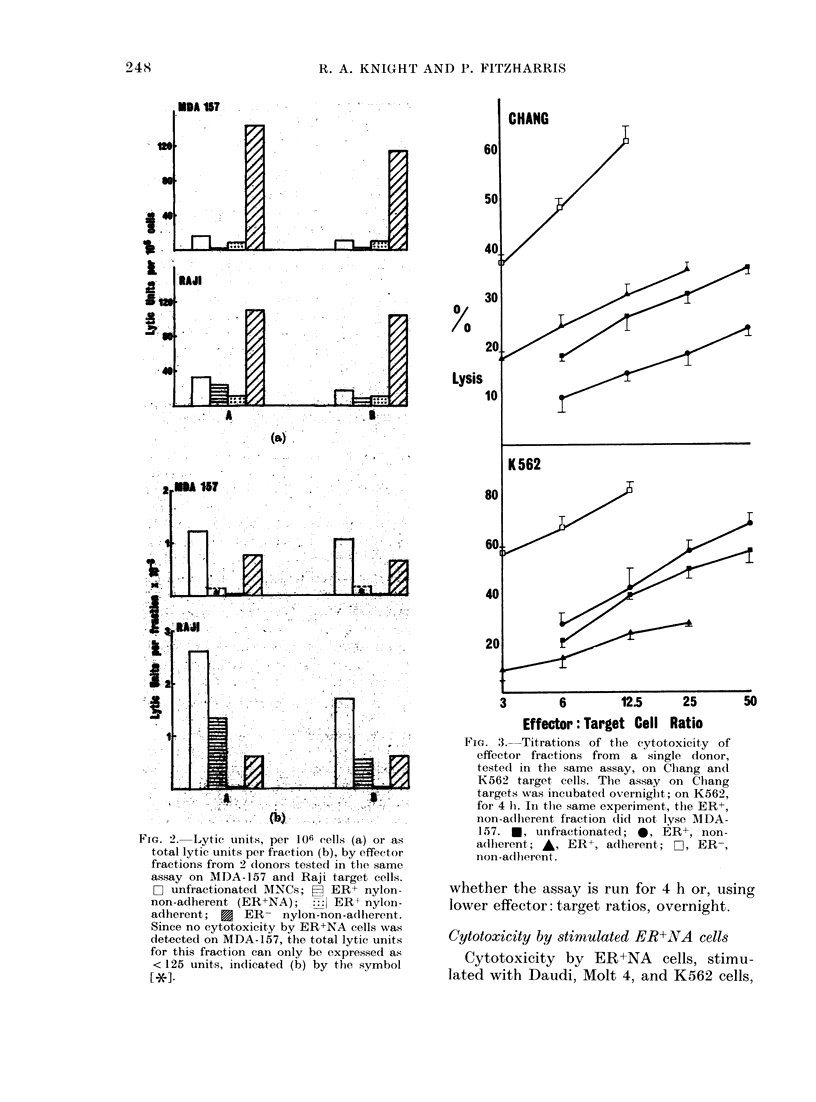
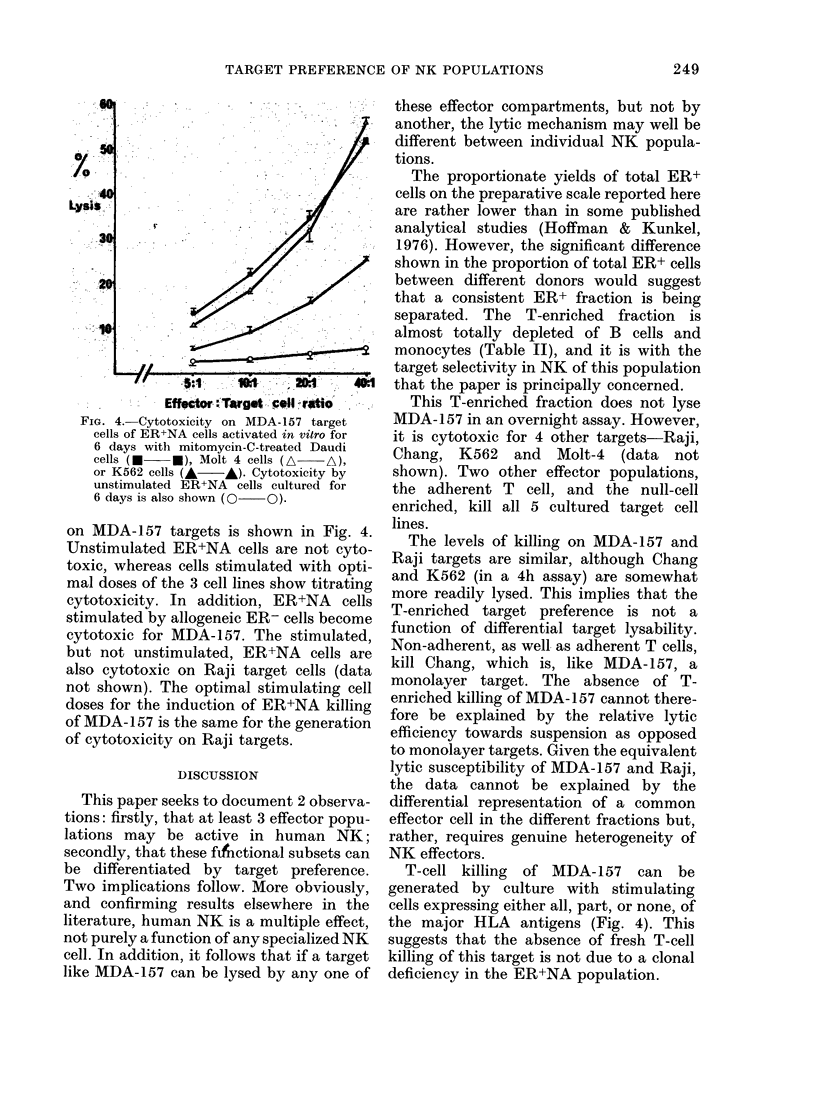
Three populations active in human spontaneous cytotoxicity have been identified. Two of these are E-rosette positive, and differ in their adherence to nylon wool. The third is E-rosette negative. The E-rosette positive fraction which does not adhere to nylon consistently does not lyse a breast-cancer-derived target, MDA-157. When tested simultaneously on 4 other tumour target cells lines--Raji, Chang, K562 and Molt 4--however, all three populations are cytolytic. The MDA-157 target is consistently lysed by a nylon-adherent T-cell fraction, irrespective of whether the E rosettes are formed under optimal or the limiting conditions giving only "high-affinity" T cells. The observation that a given effector fraction can lyse one target but not another, whereas other fractions are cytolytic on both, implies that different targets may differentiate effector populations differing in their lytic mechanism.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakács T., Gergely P., Cornain S., Klein E. Characterization of human lymphocyte subpopulations for cytotoxicity against tumor-derived monolayer cultures. Int J Cancer. 1977 Apr 15;19(4):441–449. doi: 10.1002/ijc.2910190402. [DOI] [PubMed] [Google Scholar]
- Beverley P., Knight D. Killing comes naturally. Nature. 1979 Mar 8;278(5700):119–120. doi: 10.1038/278119a0. [DOI] [PubMed] [Google Scholar]
- Bolhuis R. L., Schuit H. R., Nooyen A. M., Ronteltap C. P. Characterization of natural killer (NK) cells and killer (K) cells in human blood: discrimination between NK and K cell activities. Eur J Immunol. 1978 Oct;8(10):731–740. doi: 10.1002/eji.1830081012. [DOI] [PubMed] [Google Scholar]
- CHANG R. S. M. Continuous subcultivation of epithelial-like cells from normal human tissues. Proc Soc Exp Biol Med. 1954 Nov;87(2):440–443. doi: 10.3181/00379727-87-21406. [DOI] [PubMed] [Google Scholar]
- Cannon G. B., Bonnard G. D., Djeu J., West W. H., Herberman R. B. Relationship of human natural lymphocyte-mediated cytotoxicity to cytotoxicity of breast-cancer-derived target cells. Int J Cancer. 1977 Apr 15;19(4):487–497. doi: 10.1002/ijc.2910190409. [DOI] [PubMed] [Google Scholar]
- Grossi C. E., Webb S. R., Zicca A., Lydyard P. M., Moretta L., Mingari M. C., Cooper M. D. Morphological and histochemical analyses of two human T-cell subpopulations bearing receptors for IgM or IgG. J Exp Med. 1978 May 1;147(5):1405–1417. doi: 10.1084/jem.147.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Fernandes G., Nair M., Good R. A. Spontaneous and antibody-dependent cell-mediated cytotoxicity by human T cell subpopulations. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5137–5141. doi: 10.1073/pnas.75.10.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberman R. B., Holden H. T. Natural cell-mediated immunity. Adv Cancer Res. 1978;27:305–377. doi: 10.1016/s0065-230x(08)60936-7. [DOI] [PubMed] [Google Scholar]
- Hersey P., Edwards A., Edwards J., Adams E., Milton G. W., Nelson D. S. Specificity of cell-mediated cytotoxicity against human melanoma lines: evidence for "non-specific" killing by activated T-cells. Int J Cancer. 1975 Jul 15;16(1):173–183. doi: 10.1002/ijc.2910160119. [DOI] [PubMed] [Google Scholar]
- Jondal M., Pross H. Surface markers on human b and t lymphocytes. VI. Cytotoxicity against cell lines as a functional marker for lymphocyte subpopulations. Int J Cancer. 1975 Apr 15;15(4):596–605. doi: 10.1002/ijc.2910150409. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kall M. A., Koren H. S. Heterogeneity of human natural killer cell populations. Cell Immunol. 1978 Sep 15;40(1):58–68. doi: 10.1016/0008-8749(78)90315-5. [DOI] [PubMed] [Google Scholar]
- Kay H. D., Bonnard G. D., West W. H., Herberman R. B. A functional comparison of human Fc-receptor-bearing lymphocytes active in natural cytotoxicity and antibody-dependent cellular cytotoxicity. J Immunol. 1977 Jun;118(6):2058–2066. [PubMed] [Google Scholar]
- Klein G., Pearson G., Nadkarni J. S., Nadkarni J. J., Klein E., Henle G., Henle W., Clifford P. Relation between Epstein-Barr viral and cell membrane immunofluorescence of Burkitt tumor cells. I. Dependence of cell membrane immunofluorescence on presence of EB virus. J Exp Med. 1968 Nov 1;128(5):1011–1020. doi: 10.1084/jem.128.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide Y., Takasugi M. Determination of specificity in natural cell-mediated cytotoxicity by natural antibodies. J Natl Cancer Inst. 1977 Oct;59(4):1099–1105. doi: 10.1093/jnci/59.4.1099. [DOI] [PubMed] [Google Scholar]
- Koren H. S., Amos D. B., Buckley R. H. Natural killing in immunodeficient patients. J Immunol. 1978 Mar;120(3):796–799. [PubMed] [Google Scholar]
- Koren H. S., Williams M. S. Natural killing and antibody-dependent cellular cytotoxicity are mediated by different mechanisms and by different cells. J Immunol. 1978 Nov;121(5):1956–1960. [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Cytotoxicity of a factor isolated from human spleen. J Natl Cancer Inst. 1973 Feb;50(2):535–538. doi: 10.1093/jnci/50.2.535. [DOI] [PubMed] [Google Scholar]
- Minowada J., Onuma T., Moore G. E. Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J Natl Cancer Inst. 1972 Sep;49(3):891–895. [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- PULVERTAFT J. V. A STUDY OF MALIGNANT TUMOURS IN NIGERIA BY SHORT-TERM TISSUE CULTURE. J Clin Pathol. 1965 May;18:261–273. doi: 10.1136/jcp.18.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter M. R., Moore M. Natural cytotoxic reactivity of human lymphocyte subpopulations. Immunology. 1979 May;37(1):187–194. [PMC free article] [PubMed] [Google Scholar]
- Pross H. F., Jondal M. Cytotoxic lymphocytes from normal donors. A functional marker of human non-T lymphocytes. Clin Exp Immunol. 1975 Aug;21(2):226–235. [PMC free article] [PubMed] [Google Scholar]
- Shaw S., Pichler W. J., Nelson D. L. Fc receptors on human T-lymphocytes. III. Characterization of subpopulations involved in cell-mediated lympholysis and antibody-dependent cellular cytotoxicity. J Immunol. 1979 Feb;122(2):599–604. [PubMed] [Google Scholar]
- Takasugi M., Mickey M. R., Terasaki P. I. Reactivity of lymphocytes from normal persons on cultured tumor cells. Cancer Res. 1973 Nov;33(11):2898–2902. [PubMed] [Google Scholar]
- Vessella R. L., Gormus B. J., Lange P. H., Kaplan M. E. Heterogeneity among human lymphocyte effector cells mediating spontaneous lymphocyte-mediated cytotoxicity. Int J Cancer. 1978 May 15;21(5):594–603. doi: 10.1002/ijc.2910210509. [DOI] [PubMed] [Google Scholar]
- West W. H., Boozer R. B., Herberman R. B. Low affinity E-rosette formation by the human K cell. J Immunol. 1978 Jan;120(1):90–95. [PubMed] [Google Scholar]
- West W. H., Cannon G. B., Kay H. D., Bonnard G. D., Herberman R. B. Natural cytotoxic reactivity of human lymphocytes against a myeloid cell line: characterization of effector cells. J Immunol. 1977 Jan;118(1):355–361. [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- de Vries J. E., Cornain S., Rumke P. Cytotoxity of non-T versus T-lymphocytes from melanoma patients and healthy donors on short- and long-term cultured melanoma cells,. Int J Cancer. 1974 Oct 15;14(4):427–434. doi: 10.1002/ijc.2910140402. [DOI] [PubMed] [Google Scholar]


