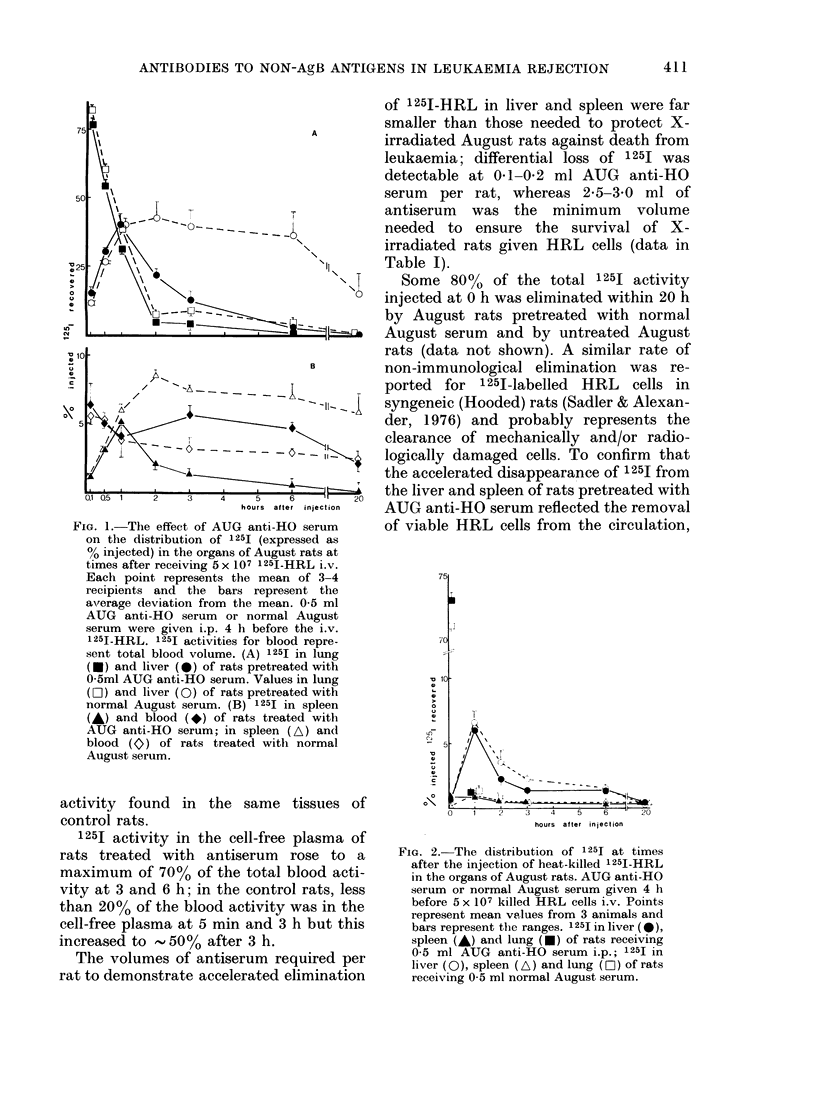
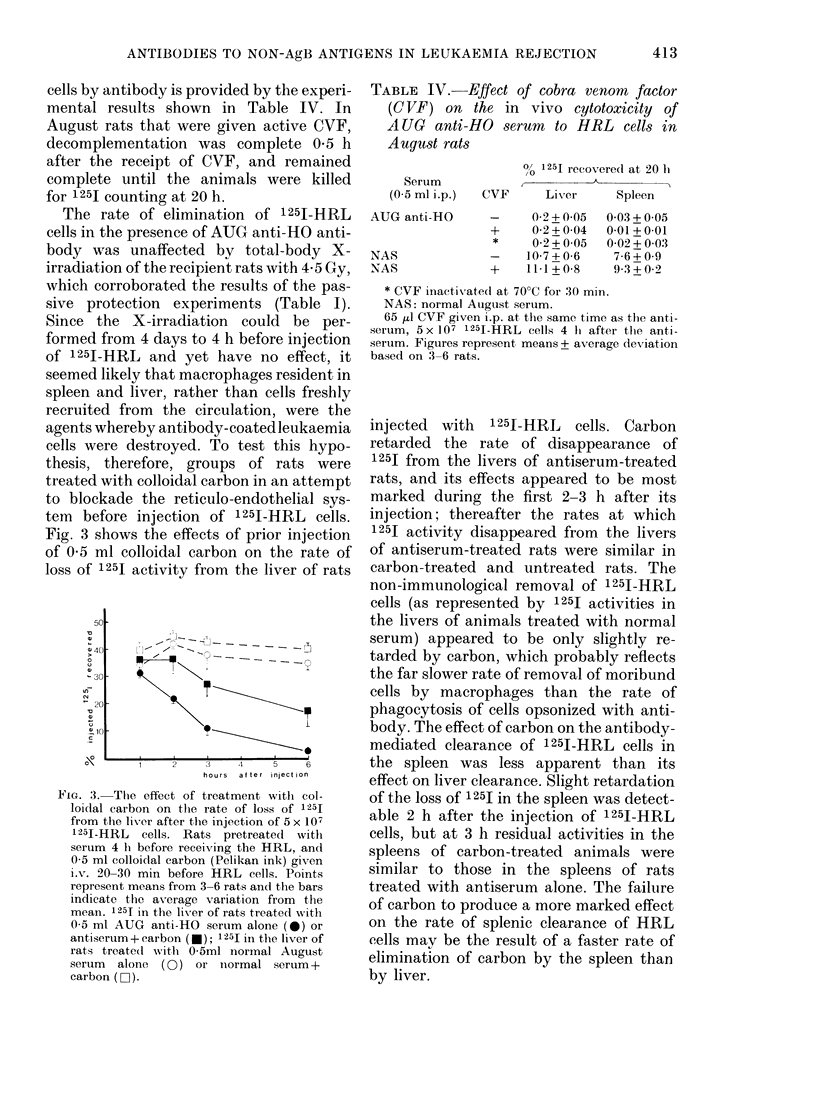
Abstract
The August and Hooded rat strains are compatible at the major histocompatibility locus (both are AgB5 or Rtlc). Antisera against the minor histocompatibility antigens of Hooded rats were raised by immunizing August rats with grafts of tumours or normal tissue. Such antisera, if transferred to normal unimmunized August rats, cause them to reject i.v. administered Hooded rat leukemia (HRL) cells within a few hours, and X-irradiated August rats, for whom a graft of HRL is lethal, can survive indefinitely if pretreated with the antiserum. The distribution of 125I-labelled HRL cells in the tissues of August rats was followed at times after their injection, and it was found that, in the presence of antiserum, i.v. administered leukaemic cells are rapidly destroyed in the liver and spleen. The active component of the antiserum is IgG antibody, and its action is independent of the lytic elements of complement. Antibody-mediated splenic and hepatic clearance of the leukaemia cells is unaffected by total-body X-irradiation but reduced by treating the rats with colloidal carbon. The data are consistent with the hypothesis that the rejection of HRL across the histocompatibility barrier studied is, in the presence of antibody, effected by immunophagocytosis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander P., Hall J. G. The role of immunoblasts in host resistance and immunotherapy of primary sarcomata. Adv Cancer Res. 1970;13:1–37. doi: 10.1016/s0065-230x(08)60162-1. [DOI] [PubMed] [Google Scholar]
- Anderson R. E., Warner N. L. Ionizing radiation and the immune response. Adv Immunol. 1976;24:215–335. doi: 10.1016/s0065-2776(08)60331-4. [DOI] [PubMed] [Google Scholar]
- Howard J. C., Scott D. W. The identification of sera distinguishing marrow-derived and thymus-derived lymphocytes in the rat thoracic duct. Immunology. 1974 Nov;27(5):903–922. [PMC free article] [PubMed] [Google Scholar]
- Lachmann P. J., Halbwachs L., Gewurz A., Gewurz H. Purification of cobra venom factor from phospholipase A contaminant. Immunology. 1976 Dec;31(6):961–968. [PMC free article] [PubMed] [Google Scholar]
- Sadler T. E., Alexander P. Trapping and destruction of blood-borne syngeneic leukaemia cells in lung, liver and spleen of normal and leukaemic rats. Br J Cancer. 1976 May;33(5):512–520. doi: 10.1038/bjc.1976.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H. S., Hayden M. L., Gately C. L. Antibody-mediated suppression of lymphoma: participation of platelets, lymphocytes, and nonphagocytic macrophages. Proc Natl Acad Sci U S A. 1974 Jan;71(1):163–166. doi: 10.1073/pnas.71.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrathmell A. B. The growth patterns of two transplantable acute leukaemias of spontaneous origin in rats. Br J Cancer. 1976 Feb;33(2):172–180. doi: 10.1038/bjc.1976.22. [DOI] [PMC free article] [PubMed] [Google Scholar]


