Abstract
Helicobacter pylori infection, which is always associated with gastritis, can progress to ulceration or malignancy. The diversity in clinical outcomes is partly attributed to the expression of virulence factors and adhesins by H. pylori. However, H. pylori may not have to adhere to the epithelium to cause gastritis. We hypothesize that outer membrane vesicles (OMV), which are constantly shed from the surface of H. pylori, play a role as independent activators of host cell responses. In this study, we found that low doses of OMV from cag PAI+ toxigenic and cag PAI− nontoxigenic strains increased proliferation of AGS gastric epithelial cells. At higher doses, we detected growth arrest, increased toxicity, and interleukin-8 (IL-8) production. The only strain differences detected were vacuolation with the toxigenic strain and higher levels of IL-8 production with OMV from the cag PAI− nontoxigenic strain. In summary, we suggest that constitutively shed OMV play a role in promoting the low-grade gastritis associated with H. pylori infection.
Gastric carriage of Helicobacter pylori occurs in more than one-half of all humans (47) and is always associated with gastritis, the host inflammatory response to colonization (5). In most infected persons, H. pylori persists in the absence of clinical illness, suggesting that there is minimal damage to gastric epithelial cells. However, variation among H. pylori strains coupled with diversity in the individual hosts and/or environmental factors can result in upset of this equilibrium (7). As a result, up to 10% of H. pylori-infected individuals are at risk for the development of disease, including peptic ulcer disease or gastric cancer (8).
H. pylori strains express two bacterial virulence factors, CagA and VacA, which have a direct effect on epithelial cells. Recent studies have shown that nearly all strains produce a VacA protein (19), whereas the proportion of strains that possess cagA is dependent upon the geographic region studied (51). CagA is encoded in a 37-kb pathogenicity island (PAI) (10), which also includes components of a type IV secretion system that translocates the CagA protein into the epithelial cells, where it is tyrosine phosphorylated (45, 63). VacA is frequently coexpressed with CagA, but it is encoded outside the PAI. It binds to high-affinity cell surface receptors (39) and is internalized by gastric epithelial cells (22). Once VacA is inside a cell, it induces cytoplasmic vacuolation (36) and cellular detachment (20). Studies have shown that intracellular CagA (57) and VacA (46) are independently associated with rearrangements of the cell cytoskeleton. Moreover, both virulence factors interfere with the normal rate of epithelial cell proliferation (54, 62) and can trigger apoptosis (15, 21, 34, 35, 49).
H. pylori cag PAI+ strains also induce the secretion of a range of inflammatory mediators by gastric epithelial cells. One prominent example is interleukin-8 (IL-8) (16), which is a potent neutrophil attractant and activator (12), and subjects infected with cag PAI+ strains have a heightened inflammatory response (50). IL-8 expression and release occur upon attachment of bacteria to the cell surface (55), and this is associated with activation of the cellular NF-κB transcription factor (59).
H. pylori can bind to epithelial cells via multiple bacterial surface components (26, 41), and evidence from animal models suggests that adherence is a contributing factor in H. pylori-associated disease (24). Although a small proportion of organisms may adhere to epithelial cells, many H. pylori cells are also found within the mucus layer (6). Gastritis and epithelial cell damage, which are initiated by the release of bacterial virulence factors, might therefore be induced in the absence of bacterial attachment to the epithelium. The detection of urease (37) and VacA (18) within the gastric mucosa supports the hypothesis that secreted bacterial products play a role in these processes.
Our research has demonstrated that H. pylori sheds outer membrane vesicles (OMV) in vitro and in vivo (29, 32). These vesicles contain proteins, lipopolysaccharide (LPS) (29), and lipoproteins (30), and gastric epithelial cells in situ (18) can internalize OMV. We propose that OMV modulate epithelial cell function independent of H. pylori adherence to the gastric epithelium. To investigate this hypothesis, we studied the effect of H. pylori OMV on cultured gastric epithelial cells.
MATERIALS AND METHODS
Bacterial strains.
Two well-characterized H. pylori clinical isolates were used in this study. OMV from H. pylori 60190 (= ATCC 49503), a wild-type cag PAI+ toxigenic strain (3, 14), were compared with OMV produced by the wild-type cag PAI− H. pylori strain Tx-30a (= ATCC 51932). which produces a VacA protein (3) that fails to induce cell vacuolation in vitro (36). The bacteria were grown in brucella broth base (Difco, Detroit, Mich.) supplemented with 5% fetal bovine serum (Gibco BRL, Arkland, New Zealand) for 72 h at 37°C under microaerobic conditions with constant rotation (120 rpm).
Preparation of OMV.
At 72 h, bacteria were removed by centrifugation (10,000 × g, 15 min, 4°C), and the supernatants were ultracentrifuged (100,000 × g, 2 h, 4°C) to recover OMV, as described previously (32). After two washes with phosphate-buffered saline (PBS), aliquots of OMV preparations were overlaid onto carbon-colloidin-coated mesh grids, negatively stained with 1% aqueous phosphotungstic acid (pH 7.0), and examined by electron microscopy to confirm the absence of whole cells and flagella. After OMV were assayed to determine the protein concentration (38), they were stored at −20°C until they were used.
Cell culture.
Cells of AGS (= ATCC CRL-1739), a human gastric epithelial cell line, were cultured in filter-sterilized F-12 nutrient mixture (HAM) (Invitrogen, Auckland, New Zealand) supplemented with 10% (vol/vol) fetal bovine serum and 1% (vol/vol) penicillin-streptomycin-glutamine supplement. Cell monolayers were cultured on petri dishes at 37°C in a humidified atmosphere consisting of 5% CO2 in air.
Cell proliferation.
The effect of OMV on proliferation of AGS gastric epithelial cells was determined by using a colorimetric assay that examined the incorporation of bromodeoxyuridine (BrdU) into the cellular DNA of proliferating cells (Roche Diagnostics, Mannheim, Germany). AGS cells (1 × 104 cells) were cultured overnight in 96-well plates and then incubated for an additional 24 h in the presence of OMV. The BrdU assay was performed as recommended by the manufacturer. Briefly, a BrdU labeling solution was added to individual wells for 2 h. Following removal of the medium, a fixative solution was added to each well for 30 min at room temperature and then replaced with BrdU antibody (1:100 dilution) for 90 min. After this the cells were washed three to five times with PBS before substrate was added. The absorbance at 370 nm was monitored for 30 min with a Spectra Max plate reader. The level of BrdU incorporation was calculated from the slope of the linear portion of the graph.
Cell viability.
Plasma membrane integrity was monitored by propidium iodide staining. Following 48 h of incubation with H. pylori OMV, culture media from two duplicate wells were removed and pooled. The adherent AGS cells were detached with 1% trypsin, the wells were washed with PBS, and the combined fractions were added to the pooled media. The cells were incubated with 2 μg of propidium iodide for 10 min, and the fluorescence of 10,000 cells was measured with a bivariate flow cytometer (Becton Dickinson, Mountain View, Calif.). Cytotoxicity was expressed as the percentage of propidium iodide-positive cells.
Cell vacuolation.
The effect of OMV on intracellular vacuolation was visualized by light microscopy at 24 h.
IL-8 expression by AGS cells.
The amount of IL-8 secreted into cell culture medium following 24 h of incubation with OMV was determined by an enzyme-linked immunosorbent assay (ELISA) by using a Quantikine kit (R&D Systems, Minneapolis, Minn.) according to the manufacturer's instructions.
Statistical analyses.
The effects of strain, dose, and length of incubation of OMV on proliferation, cytotoxicity, and IL-8 production were evaluated by using a factorial analysis of variance. When the analyses indicated that there were significant effects, the effects were examined further by using Fisher's least-significant-difference test. The IL-8 measurements were not normally distributed and were therefore loge transformed prior to analysis.
RESULTS
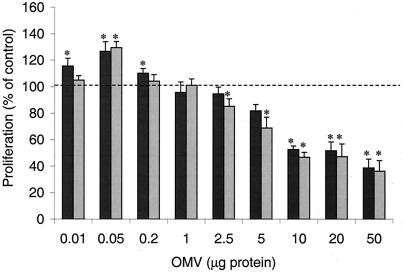
AGS gastric epithelial cells were incubated with H. pylori OMV for 24 h, and the rate of cell proliferation was measured. The lowest doses of OMV caused an increase in proliferation, and the maximal increase was a 25 to 30% increase after addition of 50 ng of OMV (Fig. 1). At OMV concentrations of 5 μg/well and higher there was a significant decrease in the rate of proliferation (Fig. 1). OMV from cag PAI+ toxigenic and cag PAI− nontoxigenic strains had similar effects on cell proliferation (Fig. 1).
FIG. 1.
Effect of H. pylori OMV on gastric epithelial cell proliferation. AGS cells were grown alone or in the presence of different concentrations of H. pylori OMV from either a cag PAI+ toxigenic strain (dark gray bars) or a cag PAI− nontoxigenic strain (light gray bars). Cell proliferation was assessed by examining the incorporation of BrdU 24 h after inoculation, and the results are expressed as percentages of the incorporation observed in untreated cells. The data are means ± standard errors for three to six independent experiments performed in triplicate. An asterisk indicates that the results for a treatment are statistically significantly different (P < 0.05) from the results for untreated cells.
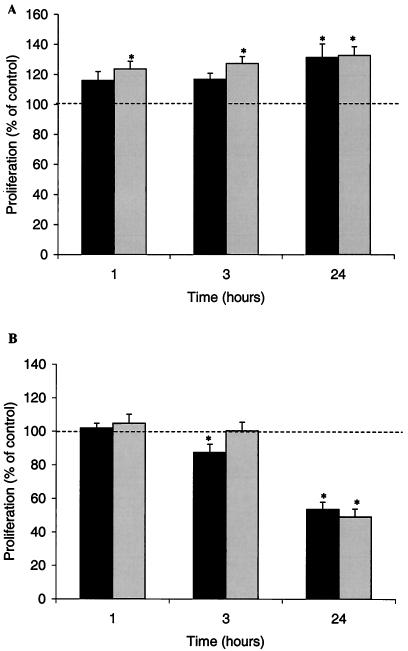
In order to determine the lead time for an observable effect of OMV on epithelial cell proliferation, we investigated the length of time that the OMV took to initiate a response. A low dose of OMV triggered an immediate increase in the rate of proliferation (1 h of incubation and then 2 h with BrdU) (Fig. 2A). The growth arrest observed with higher concentrations of OMV took significantly longer, and the maximal effect was not seen until 24 h (Fig. 2B).
FIG. 2.
Time course of H. pylori OMV effect on epithelial cell proliferation. AGS cells were treated with 0.05 μg (A) or 10 μg (B) of OMV from a cag PAI+ toxigenic strain (dark gray bars) or a cag PAI− nontoxigenic strain (light gray bars) of H. pylori for either 24, 3, or 1 h before the rate of proliferation was measured. The results are expressed as percentages of the values obtained for untreated cells. The data are means ± standard errors for five (A) or two (B) independent experiments performed in triplicate. An asterisk indicates that the results for a treatment are statistically significantly different (P < 0.05) from the results for untreated cells.
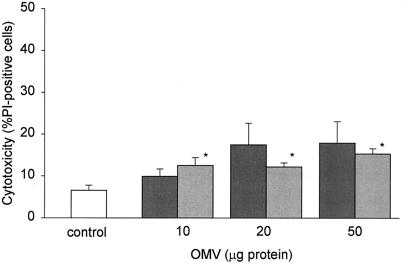
To test whether the decreased proliferation at high doses of OMV was associated with toxicity, we measured cell viability by the propidium iodide dye exclusion assay. There was a statistically significant increase in toxicity at the highest doses of H. pylori OMV (10 to 50 μg/ml), but at these doses the level of epithelial cell death was <20% (Fig. 3). As with proliferation, there were no strain-dependent differences.
FIG. 3.
Effect of H. pylori OMV on gastric epithelial cell viability. AGS cells were grown alone or in the presence of 10, 20, or 50 μg of H. pylori OMV from a cag PAI+ toxigenic strain (dark gray bars) or a cag PAI− nontoxigenic strain (light gray bars). Cell viability was assessed by propidium iodide (PI) staining and flow cytometry after 24 h. Cytotoxicity is expressed as the percentage of propidium iodide-positive cells in each population. The data are means ± standard errors for five independent experiments. An asterisk indicates that the results for a treatment are statistically significantly different (P < 0.05) from the results for untreated cells.
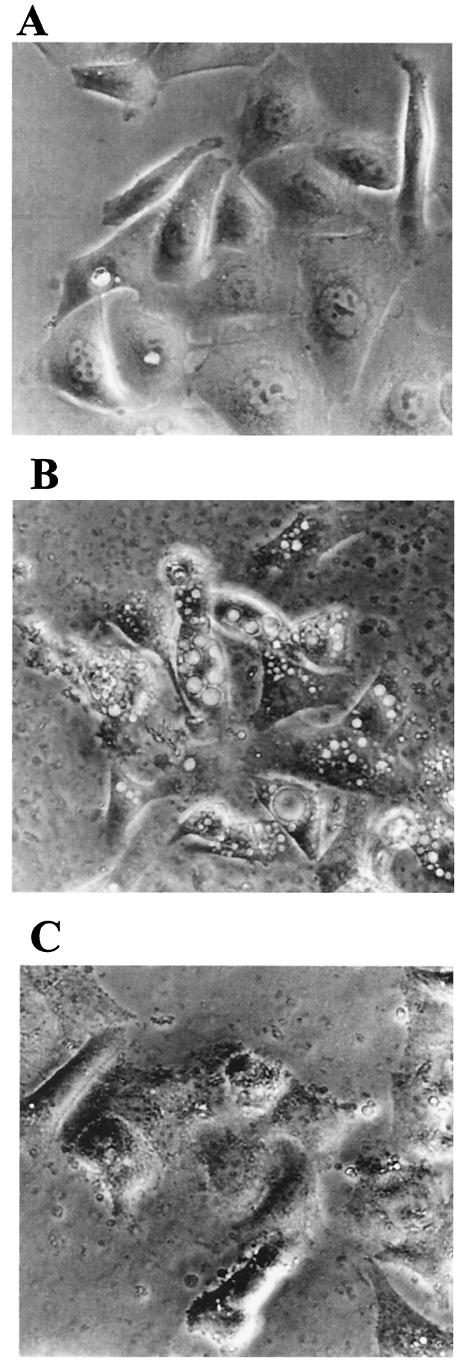
AGS cells are sensitive to the VacA cytotoxin (61), and VacA has been shown to be associated with OMV from the toxigenic strain (29). We observed vacuolation in cells treated with OMV from the toxigenic H. pylori strain (Fig. 4). In contrast, no intracellular vacuolation was observed with OMV from the nontoxigenic strain, although at the high dose (50 μg), a change in cell morphology was evident (Fig. 4). Some vacuolation was observed with 10 μg of 60190 OMV (data not shown), but this response was less pronounced than the vacuolation observed with 50 μg.
FIG.4.
Vacuolation in gastric epithelial cells treated with OMV. AGS cells were grown alone (A) or in the presence of H. pylori OMV (50 μg of protein) from a cag PAI+ toxigenic strain (B) or a cag PAI− nontoxigenic strain (C). Cell vacuolation was assessed by light microscopy after 24 h. The photographs are from a representative experiment.
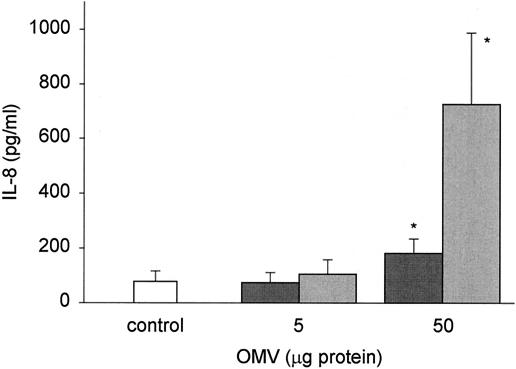
Exposure of AGS cells to 50 ng (results not shown) and 5 μg (Fig. 5) of OMV did not induce any significant increase in IL-8 expression above the basal level. Conversely, higher doses of OMV (50 μg) were associated with significant cellular expression of the proinflammatory cytokine IL-8 (Fig. 5). The OMV from the cag PAI− nontoxigenic strain induced more IL-8 production by AGS cells than the OMV from the cag PAI+ toxigenic strain induced (Fig. 5).
FIG. 5.
H. pylori OMV stimulate IL-8 production from gastric epithelial cells. AGS cells were grown alone or in the presence of different concentrations of H. pylori OMV (5 to 50 μg of protein) from either a cag PAI+ toxigenic strain (dark gray bars) or a cag PAI− nontoxigenic strain (light gray bars). Culture medium was collected after 24 h, and the IL-8 concentration was measured by an ELISA. The data are means ± standard errors for four independent experiments. An asterisk indicates an amount is statistically significantly different (P < 0.05) from the amount produced by untreated cells. There was a strain-dependent effect with 50 μg of OMV (P = 0.008).
DISCUSSION
In this study, we found that H. pylori OMV have a direct effect on cultured gastric epithelial cells, similar to the effect described for whole bacteria. H. pylori OMV are small, circular structures with an intact outer membrane (29) that are constantly shed from the surface of H. pylori. They are composed of protein, LPS (29), and lipoproteins (30). VacA is present (18, 29), but there is no evidence of CagA or urease B or its associated chaperonin (HspB) in the vesicles (29). Depending on the dose of OMV, we detected enhanced or decreased cell proliferation, vacuolation, a loss of cell viability, and production of the proinflammatory cytokine IL-8. These observations support the hypothesis that the effect of H. pylori on gastric epithelial cells is not completely dependent on bacterial attachment.
OMV from both the cag PAI+ toxigenic strain and the cag PAI− nontoxigenic strain used in this study enhanced proliferation at low doses, while higher doses resulted in growth arrest. These findings are similar to those obtained after addition of whole bacteria or extracts to cultured epithelial cells (17, 33, 54, 62, 66). The mechanisms of OMV action are not clear, but we presume that in the first instance contact between OMV and host cells is necessary. Binding of OMV to the cell surface may promote enhanced activity of growth factor receptors at lower doses and blockage of these pathways at higher doses. However, Fiocca et al. reported that H. pylori OMV are internalized into epithelial cells (18). This process, which may be receptor mediated, has the potential to modulate cell-signaling pathways.
Activity of the vacuolating cytotoxin VacA provides an alternate pathway for inhibition of cell proliferation (54), yet other studies support our observation that growth arrest is not strain related (33, 62, 66). OMV from the toxigenic H. pylori strain used in this study induced prominent cytoplasmic vacuolation in the AGS cells, typical of the VacA cytotoxin (36), and this was associated with a small but significant decrease in AGS cell viability. We observed no vacuolation of AGS cells cultured with OMV from the nontoxigenic H. pylori strain. In spite of this, these vesicles also induced growth arrest and a small decrease in AGS cell viability. Essentially all H. pylori strains possess the vacA gene (3), and evidence that the mechanism involved in VacA-associated apoptosis is distinct from vacuolating cytoxocity (34) suggested that there is a link between vesicle-associated VacA and our observation that there is reduced cell viability, even in the absence of vacuolation. However, toxigenic strains differ not only genotypically (3) but also at the level of vacA transcription (19) and VacA expression (13). We detected VacA in OMV from the nontoxigenic strain, but the amount was considerably smaller than the amount present in toxigenic OMV (31). This study and other studies (64, 66) suggest that outer membrane factors other than VacA are involved in growth arrest.
We found that coincubation of H. pylori OMV with AGS cells induced a significant increase in the expression of IL-8, a potent neutrophil-activating chemokine (12). Direct contact between H. pylori and host cells appears to be critical for initiation of NF-κB activation, which mediates an increase in IL-8 expression (1, 55, 58). There have been a number of studies in which the workers found that the regulation of IL-8 production is associated with H. pylori virulence (16, 25, 50). This virulence is associated with the presence of the cag PAI in these strains (11). When there is contact with gastric epithelial cells, cag-encoded proteins activate multiple signal transduction cascades that regulate IL-8 secretion (48), including NF-κB (23) and AP-1 (1) activation. Immunoblotting has shown that OMV from cag PAI+ strains do not contain CagA (29); however, whether other components of the cag PAI are present has yet to be established. We found that cag PAI− nontoxigenic OMV induced the highest level of IL-8 production by AGS cells. Other workers have observed IL-8 production following incubation of epithelial cells with cag PAI− H. pylori strains (60, 67). Together, these observations support the hypothesis that the cag PAI is not the sole factor involved in IL-8 production.
In vitro studies have shown that water-extracted surface proteins (25, 27, 55) elicit IL-8 secretion by epithelial cells. More recently, the oipA gene, which encodes an outer membrane protein, has been has been found to induce this effect (68). However, no causative link has been found between knockout mutants of this gene (2, 44) or other outer membrane proteins and adhesins (25, 44, 68) and IL-8 induction.
As an alternative mechanism, H. pylori LPS has been shown to induce IL-8 expression by gastric epithelial cells (25). Epithelial cells possess a set of evolutionarily conserved membrane-bound Toll-like receptors (TLRs) which act as pattern recognition receptors for conserved pathogen-associated molecular patterns. These TLRs play an essential role in the initiation of cellular innate immune responses via the expression of proinflammatory cytokines, such as IL-8 (40). In gram-negative bacteria, this is primarily through TLR4 recognition of a pathogen-associated molecular pattern in the hydrophobic lipid A portion of LPS (65). H. pylori LPS is generally considered to be a less toxic virulence factor (43, 52) because of an altered phosphorylation pattern within lipid A (42). Recently, however, H. pylori LPS has been shown to activate NF-κB in guinea pig gastric cells via a TLR4 receptor (28), which correlates with a previous observation that C3H/HeJ mice (which carry a point mutation in the TLR4 gene locus that can eliminate LPS-induced cytokine responses [53]) fail to mount a cellular response to long-term Helicobacter infection (56). AGS cells have been shown to express TLR4, and while this receptor was not detected in primary gastric epithelial cells isolated from H. pylori-negative individuals (4), other workers have observed increased TLR4 expression in intestinal epithelial cells during inflammation (9).
In summary, in this study we found that OMV, which are constantly shed from the surface of H. pylori in vivo, have a measurable effect on gastric epithelial cell responses that are not always associated with well-described virulence factors. We did not observe any significant strain differences in terms of the ability of OMV to initiate cytotoxicity in host cells or alter their rate of proliferation. There were, however, differences in epithelial cell vacuolation and epithelial cell IL-8 production. We speculate that these constitutively shed OMV-associated factors play a role in promoting host responses via low-grade gastritis to support persistence of H. pylori in the stomach.
Acknowledgments
This study was supported by grants from the Robert McClelland Trust and the Health Research Council of New Zealand.
We thank Tessa Mocatta for performing the IL-8 ELISAs, Lisa Haring for running the flow cytometer, and Chris Frampton for performing the statistical analysis.
Editor: J. D. Clements
REFERENCES
- 1.Aihara, M., D. Tsuchimoto, H. Takizawa, A. Azuma, H. Wakebe, Y. Ohmoto, K. Imagawa, M. Kikuchi, N. Mukaida, and K. Matsushima. 1997. Mechanisms involved in Helicobacter pylori-induced interleukin-8 production by a gastric cancer cell line, MKN45. Infect. Immun. 65:3218-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ando, T., R. M. Peek, Jr., Y. C. Lee, U. Krishna, K. Kusugami, and M. J. Blaser. 2002. Host cell responses to genotypically similar Helicobacter pylori isolates from United States and Japan. Clin. Diagn. Lab. Immunol. 9:167-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atherton, J. C., P. Cao, R. M. Peek, Jr., M. K. Tummuru, M. J. Blaser, and T. L. Cover. 1995. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J. Biol. Chem. 270:17771-17777. [DOI] [PubMed] [Google Scholar]
- 4.Backhed, F., B. Rokbi, E. Torstensson, Y. Zhao, C. Nilsson, D. Seguin, S. Normark, A. M. Buchan, and A. Richter-Dahlfors. 2003. Gastric mucosal recognition of Helicobacter pylori is independent of Toll-like receptor 4. J. Infect. Dis. 187:829-836. [DOI] [PubMed] [Google Scholar]
- 5.Blaser, M. J. 1992. Helicobacter pylori: its role in disease. Clin. Infect. Dis. 15:386-391. [DOI] [PubMed] [Google Scholar]
- 6.Blaser, M. J. 1995. The role of Helicobacter pylori in gastritis and its progression to peptic ulcer disease. Aliment. Pharmacol. Ther. 9:27-30. [DOI] [PubMed] [Google Scholar]
- 7.Blaser, M. J., and D. E. Berg. 2001. Helicobacter pylori genetic diversity and risk of human disease. J. Clin. Investig. 107:767-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blaser, M. J., and J. Parsonnet. 1994. Parasitism by the “slow” bacterium Helicobacter pylori leads to altered gastric homeostasis and neoplasia. J. Clin. Investig. 94:4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cario, E., and D. K. Podolsky. 2000. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect. Immun. 68:7010-7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Censini, S., C. Lange, Z. Xiang, J. E. Crabtree, P. Ghiara, M. Borodovsky, R. Rappuoli, and A. Covacci. 1996. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc. Natl. Acad. Sci. USA 93:14648-14653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Censini, S., M. Stein, and A. Covacci. 2001. Cellular responses induced after contact with Helicobacter pylori. Curr. Opin. Microbiol. 4:41-46. [DOI] [PubMed] [Google Scholar]
- 12.Chensue, S. W. 2001. Molecular machinations: chemokine signals in host-pathogen interactions. Clin. Microbiol. Rev. 14:821-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cover, T. L., P. Cao, C. D. Lind, K. T. Tham, and M. J. Blaser. 1993. Correlation between vacuolating cytotoxin production by Helicobacter pylori isolates in vitro and in vivo. Infect. Immun. 61:5008-5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cover, T. L., C. P. Dooley, and M. J. Blaser. 1990. Characterization of and human serologic response to proteins in Helicobacter pylori broth culture supernatants with vacuolizing cytotoxin activity. Infect. Immun. 58:603-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cover, T. L., U. S. Krishna, D. A. Israel, and R. M. Peek, Jr. 2003. Induction of gastric epithelial cell apoptosis by Helicobacter pylori vacuolating cytotoxin. Cancer Res. 63:951-957. [PubMed] [Google Scholar]
- 16.Crabtree, J. E., Z. Xiang, I. J. Lindley, D. S. Tompkins, R. Rappuoli, and A. Covacci. 1995. Induction of interleukin-8 secretion from gastric epithelial cells by a cagA negative isogenic mutant of Helicobacter pylori. J. Clin. Pathol. 48:967-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan, X. G., D. Kelleher, X. J. Fan, H. X. Xia, and P. W. Keeling. 1996. Helicobacter pylori increases proliferation of gastric epithelial cells. Gut 38:19-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiocca, R., V. Necchi, P. Sommi, V. Ricci, J. Telford, T. L. Cover, and E. Solcia. 1999. Release of Helicobacter pylori vacuolating cytotoxin by both a specific secretion pathway and budding of outer membrane vesicles. Uptake of released toxin and vesicles by gastric epithelium. J. Pathol. 188:220-226. [DOI] [PubMed] [Google Scholar]
- 19.Forsyth, M. H., J. C. Atherton, M. J. Blaser, and T. L. Cover. 1998. Heterogeneity in levels of vacuolating cytotoxin gene (vacA) transcription among Helicobacter pylori strains. Infect. Immun. 66:3088-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujikawa, A., D. Shirasaka, S. Yamamoto, H. Ota, K. Yahiro, M. Fukada, T. Shintani, A. Wada, N. Aoyama, T. Hirayama, H. Fukamachi, and M. Noda. 2003. Mice deficient in protein tyrosine phosphatase receptor type Z are resistant to gastric ulcer induction by VacA of Helicobacter pylori. Nat. Genet. 33:375-381. [DOI] [PubMed] [Google Scholar]
- 21.Galmiche, A., J. Rassow, A. Doye, S. Cagnol, J. C. Chambard, S. Contamin, V. de Thillot, I. Just, V. Ricci, E. Solcia, E. Van Obberghen, and P. Boquet. 2000. The N-terminal 34 kDa fragment of Helicobacter pylori vacuolating cytotoxin targets mitochondria and induces cytochrome c release. EMBO J. 19:6361-6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garner, J. A., and T. L. Cover. 1996. Binding and internalization of the Helicobacter pylori vacuolating cytotoxin by epithelial cells. Infect. Immun. 64:4197-4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glocker, E., C. Lange, A. Covacci, S. Bereswill, M. Kist, and H. L. Pahl. 1998. Proteins encoded by the cag pathogenicity island of Helicobacter pylori are required for NF-κB activation. Infect. Immun. 66:2346-2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guruge, J. L., P. G. Falk, R. G. Lorenz, M. Dans, H. P. Wirth, M. J. Blaser, D. E. Berg, and J. I. Gordon. 1998. Epithelial attachment alters the outcome of Helicobacter pylori infection. Proc. Natl. Acad. Sci. USA 95:3925-3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang, J., P. W. O'Toole, P. Doig, and T. J. Trust. 1995. Stimulation of interleukin-8 production in epithelial cell lines by Helicobacter pylori. Infect. Immun. 63:1732-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ilver, D., A. Arnqvist, J. Ogren, I. M. Frick, D. Kersulyte, E. T. Incecik, D. E. Berg, A. Covacci, L. Engstrand, and T. Boren. 1998. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science 279:373-377. [DOI] [PubMed] [Google Scholar]
- 27.Kassai, K., T. Yoshikawa, N. Yoshida, A. Hashiramoto, M. Kondo, and H. Murase. 1999. Helicobacter pylori water extract induces interleukin-8 production by gastric epithelial cells. Dig. Dis. Sci. 44:237-242. [DOI] [PubMed] [Google Scholar]
- 28.Kawahara, T., Y. Kuwano, S. Teshima-Kondo, T. Kawai, T. Nikawa, K. Kishi, and K. Rokutan. 2001. Toll-like receptor 4 regulates gastric pit cell responses to Helicobacter pylori infection. J. Med. Investig. 48:190-197. [PubMed] [Google Scholar]
- 29.Keenan, J., T. Day, S. Neal, B. Cook, G. Perez-Perez, R. Allardyce, and P. Bagshaw. 2000. A role for the bacterial outer membrane in the pathogenesis of Helicobacter pylori infection. FEMS Microbiol. Lett. 182:259-264. [DOI] [PubMed] [Google Scholar]
- 30.Keenan, J., J. Oliaro, N. Domigan, H. Potter, G. Aitken, R. Allardyce, and J. Roake. 2000. Immune response to an 18-kilodalton outer membrane antigen identifies lipoprotein 20 as a Helicobacter pylori vaccine candidate. Infect. Immun. 68:3337-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keenan, J. I., and R. A. Allardyce. 2000. Iron influences the expression of Helicobacter pylori outer membrane vesicle-associated virulence factors. Eur. J. Gastroenterol. Hepatol. 12:1267-1273. [DOI] [PubMed] [Google Scholar]
- 32.Keenan, J. I., R. A. Allardyce, and P. F. Bagshaw. 1997. Dual silver staining to characterise Helicobacter spp. outer membrane components. J. Immunol. Methods 209:17-24. [DOI] [PubMed] [Google Scholar]
- 33.Knipp, U., S. Birkholz, W. Kaup, and W. Opferkuch. 1996. Partial characterization of a cell proliferation-inhibiting protein produced by Helicobacter pylori. Infect. Immun. 64:3491-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuck, D., B. Kolmerer, C. Iking-Konert, P. H. Krammer, W. Stremmel, and J. Rudi. 2001. Vacuolating cytotoxin of Helicobacter pylori induces apoptosis in the human gastric epithelial cell line AGS. Infect. Immun. 69:5080-5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le'Negrate, G., V. Ricci, V. Hofman, B. Mograbi, P. Hofman, and B. Rossi. 2001. Epithelial intestinal cell apoptosis induced by Helicobacter pylori depends on expression of the cag pathogenicity island phenotype. Infect. Immun. 69:5001-5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leunk, R. D., P. T. Johnson, B. C. David, W. G. Kraft, and D. R. Morgan. 1988. Cytotoxic activity in broth-culture filtrates of Campylobacter pylori. J. Med. Microbiol. 26:93-99. [DOI] [PubMed] [Google Scholar]
- 37.Mai, U. E., G. I. Perez-Perez, J. B. Allen, S. M. Wahl, M. J. Blaser, and P. D. Smith. 1992. Surface proteins from Helicobacter pylori exhibit chemotactic activity for human leukocytes and are present in gastric mucosa. J. Exp. Med. 175:517-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Markwell, M. A., S. M. Haas, L. L. Bieber, and N. E. Tolbert. 1978. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 87:206-210. [DOI] [PubMed] [Google Scholar]
- 39.Massari, P., R. Manetti, D. Burroni, S. Nuti, N. Norais, R. Rappuoli, and J. L. Telford. 1998. Binding of the Helicobacter pylori vacuolating cytotoxin to target cells. Infect. Immun. 66:3981-3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Medzhitov, R., and C. A. Janeway, Jr. 1997. Innate immunity: the virtues of a nonclonal system of recognition. Cell 91:295-298. [DOI] [PubMed] [Google Scholar]
- 41.Moran, A. P. 1996. Pathogenic properties of Helicobacter pylori. Scand. J. Gastroenterol. Suppl. 215:22-31. [PubMed] [Google Scholar]
- 42.Moran, A. P., I. M. Helander, and T. U. Kosunen. 1992. Compositional analysis of Helicobacter pylori rough-form lipopolysaccharides. J. Bacteriol. 174:1370-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muotiala, A., I. M. Helander, L. Pyhala, T. U. Kosunen, and A. P. Moran. 1992. Low biological activity of Helicobacter pylori lipopolysaccharide. Infect. Immun. 60:1714-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Odenbreit, S., H. Kavermann, J. Puls, and R. Haas. 2002. CagA tyrosine phosphorylation and interleukin-8 induction by Helicobacter pylori are independent from alpAB, HopZ and bab group outer membrane proteins. Int. J. Med. Microbiol. 292:257-266. [DOI] [PubMed] [Google Scholar]
- 45.Odenbreit, S., J. Puls, B. Sedlmaier, E. Gerland, W. Fischer, and R. Haas. 2000. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 287:1497-1500. [DOI] [PubMed] [Google Scholar]
- 46.Pai, R., T. L. Cover, and A. S. Tarnawski. 1999. Helicobacter pylori vacuolating cytotoxin (VacA) disorganizes the cytoskeletal architecture of gastric epithelial cells. Biochem. Biophys. Res. Commun. 262:245-250. [DOI] [PubMed] [Google Scholar]
- 47.Parsonnet, J. 1995. Bacterial infection as a cause of cancer. Environ. Health Perspect. 103(Suppl. 8):263-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peek, R. M., Jr. 2001. Helicobacter pylori strain-specific activation of signal transduction cascades related to gastric inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 280:G525-G530. [DOI] [PubMed] [Google Scholar]
- 49.Peek, R. M., Jr., M. J. Blaser, D. J. Mays, M. H. Forsyth, T. L. Cover, S. Y. Song, U. Krishna, and J. A. Pietenpol. 1999. Helicobacter pylori strain-specific genotypes and modulation of the gastric epithelial cell cycle. Cancer Res. 59:6124-6131. [PubMed] [Google Scholar]
- 50.Peek, R. M., Jr., G. G. Miller, K. T. Tham, G. I. Perez-Perez, X. Zhao, J. C. Atherton, and M. J. Blaser. 1995. Heightened inflammatory response and cytokine expression in vivo to cagA+ Helicobacter pylori strains. Lab. Investig. 73:760-770. [PubMed] [Google Scholar]
- 51.Perez-Perez, G. I., N. Bhat, J. Gaensbauer, A. Fraser, D. N. Taylor, E. J. Kuipers, L. Zhang, W. C. You, and M. J. Blaser. 1997. Country-specific constancy by age in cagA+ proportion of Helicobacter pylori infections. Int. J. Cancer 72:453-456. [DOI] [PubMed] [Google Scholar]
- 52.Perez-Perez, G. I., V. L. Shepherd, J. D. Morrow, and M. J. Blaser. 1995. Activation of human THP-1 cells and rat bone marrow-derived macrophages by Helicobacter pylori lipopolysaccharide. Infect. Immun. 63:1183-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qureshi, S. T., L. Lariviere, G. Leveque, S. Clermont, K. J. Moore, P. Gros, and D. Malo. 1999. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J. Exp. Med. 189:615-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ricci, V., C. Ciacci, R. Zarrilli, P. Sommi, M. K. Tummuru, C. Del Vecchio Blanco, C. B. Bruni, T. L. Cover, M. J. Blaser, and M. Romano. 1996. Effect of Helicobacter pylori on gastric epithelial cell migration and proliferation in vitro: role of VacA and CagA. Infect. Immun. 64:2829-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rieder, G., R. A. Hatz, A. P. Moran, A. Walz, M. Stolte, and G. Enders. 1997. Role of adherence in interleukin-8 induction in Helicobacter pylori-associated gastritis. Infect. Immun. 65:3622-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sakagami, T., J. Vella, M. F. Dixon, J. O'Rourke, F. Radcliff, P. Sutton, T. Shimoyama, K. Beagley, and A. Lee. 1997. The endotoxin of Helicobacter pylori is a modulator of host-dependent gastritis. Infect. Immun. 65:3310-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Segal, E. D., J. Cha, J. Lo, S. Falkow, and L. S. Tompkins. 1999. Altered states: involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc. Natl. Acad. Sci. USA 96:14559-14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Selbach, M., S. Moese, T. F. Meyer, and S. Backert. 2002. Functional analysis of the Helicobacter pylori cag pathogenicity island reveals both VirD4-CagA-dependent and VirD4-CagA-independent mechanisms. Infect. Immun. 70:665-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharma, S. A., M. K. Tummuru, M. J. Blaser, and L. D. Kerr. 1998. Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor-kappa B in gastric epithelial cells. J. Immunol. 160:2401-2407. [PubMed] [Google Scholar]
- 60.Sharma, S. A., M. K. Tummuru, G. G. Miller, and M. J. Blaser. 1995. Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect. Immun. 63:1681-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smoot, D. T., J. H. Resau, M. H. Earlington, M. Simpson, and T. L. Cover. 1996. Effects of Helicobacter pylori vacuolating cytotoxin on primary cultures of human gastric epithelial cells. Gut 39:795-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smoot, D. T., Z. Wynn, T. B. Elliott, C. R. Allen, G. Mekasha, T. Naab, and H. Ashktorab. 1999. Effects of Helicobacter pylori on proliferation of gastric epithelial cells in vitro. Am. J. Gastroenterol. 94:1508-1511. [DOI] [PubMed] [Google Scholar]
- 63.Stein, M., R. Rappuoli, and A. Covacci. 2000. Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation. Proc. Natl. Acad. Sci. USA 97:1263-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takagi, A., S. Watanabe, M. Igarashi, J. Koike, K. Hasumi, R. Deguchi, Y. Koga, and T. Miwa. 2000. The effect of Helicobacter pylori on cell proliferation and apoptosis in gastric epithelial cell lines. Aliment. Pharmacol. Ther. 14(Suppl. 1):188-192. [DOI] [PubMed] [Google Scholar]
- 65.Vasselon, T., and P. A. Detmers. 2002. Toll receptors: a central element in innate immune responses. Infect. Immun. 70:1033-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wagner, S., W. Beil, J. Westermann, R. P. Logan, C. T. Bock, C. Trautwein, J. S. Bleck, and M. P. Manns. 1997. Regulation of gastric epithelial cell growth by Helicobacter pylori: evidence for a major role of apoptosis. Gastroenterology 113:1836-1847. [DOI] [PubMed] [Google Scholar]
- 67.Yamaoka, Y., T. Kodama, M. Kita, J. Imanishi, K. Kashima, and D. Y. Graham. 1999. Relation between clinical presentation, Helicobacter pylori density, interleukin 1beta and 8 production, and cagA status. Gut 45:804-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamaoka, Y., D. H. Kwon, and D. Y. Graham. 2000. A M(r) 34,000 proinflammatory outer membrane protein (oipA) of Helicobacter pylori. Proc. Natl. Acad. Sci. USA 97:7533-7538. [DOI] [PMC free article] [PubMed] [Google Scholar]