Abstract
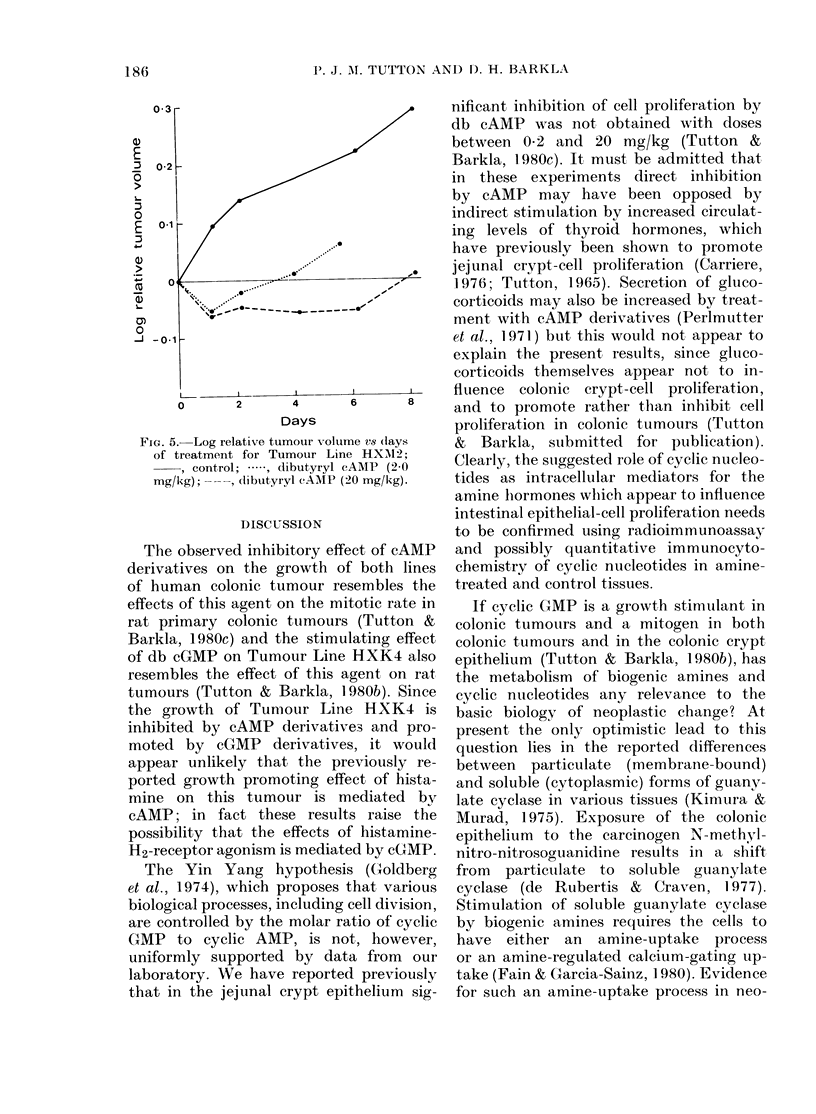
Previous studies have shown that various amine hormones are able to influence the growth rate of human colorectal carcinomas propagated as xenografts in immune-deprived mice, and it is now well known that the effects of many amine and other hormones are mediated by cyclic nucleotides, acting as second messengers within cells. In the present study the influence of various derivatives of cyclic adenosine monophosphate and cyclic guanosine monophosphate on the growth of two different lines of colorectal cancer growing in immune-deprived mice, and on the cell production rate in the colonic crypt epithelium of the rat, was assessed. Growth of each tumour line, as well as crypt-cell production, was suppressed by treatment wit N6O2' dibutyryl and N6 monobutyryl derivatives of cyclic adenosine monophosphate. Dibutyryl cyclic guanosine monophosphate, on the other hand, was found to promote the growth of Tumour HXK4 and to promote crypt cell production, but to have no significant effect on Tumour HXM2.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ash A. S., Schild H. O. Receptors mediating some actions of histamine. Br J Pharmacol Chemother. 1966 Aug;27(2):427–439. doi: 10.1111/j.1476-5381.1966.tb01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthelsen S., Pettinger W. A. A functional basis for classification of alpha-adrenergic receptors. Life Sci. 1977 Sep 1;21(5):595–606. doi: 10.1016/0024-3205(77)90066-2. [DOI] [PubMed] [Google Scholar]
- Black J. W., Duncan W. A., Durant C. J., Ganellin C. R., Parsons E. M. Definition and antagonism of histamine H 2 -receptors. Nature. 1972 Apr 21;236(5347):385–390. doi: 10.1038/236385a0. [DOI] [PubMed] [Google Scholar]
- Carriere R. M. The influence of thyroid and testicular hormones on the epithelium of crypts of Lieberkühn in the rat's intestine. Anat Rec. 1966 Dec;156(4):423–431. doi: 10.1002/ar.1091560406. [DOI] [PubMed] [Google Scholar]
- Chawla R. K., Nixon D. W., Shoji M., Rudman D. Plasma and urine cyclic guanosine 3':5'-monophosphate in disseminated cancer. Ann Intern Med. 1979 Dec;91(6):862–864. doi: 10.7326/0003-4819-91-6-862. [DOI] [PubMed] [Google Scholar]
- DeRubertis F. R., Chayoth R., Field J. B. The content and metabolism of cyclic adenosine 3', 5'-monophosphate and cyclic guanosine 3', 5'-monophosphate in adenocarcinoma of the human colon. J Clin Invest. 1976 Mar;57(3):641–649. doi: 10.1172/JCI108320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRubertis F. R., Craven P. A. Activation of the guanylate cyclase-guanosine 3'5' monophosphate system of colonic mucosa by n-methyl-n'-nitro-n-nitrosoguanidine. Cancer. 1977 Nov;40(5 Suppl):2600–2608. doi: 10.1002/1097-0142(197711)40:5+<2600::aid-cncr2820400932>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Enjalbert A., Bourgoin S., Hamon M., Adrien J., Bockaert J. Postsynaptic serotonin-sensitive adenylate cyclase in the central nervous system. I. Development and distribution of serotonin and dopamine-sensitive adenylate cyclases in rat and guinea pig brain. Mol Pharmacol. 1978 Jan;14(1):2–10. [PubMed] [Google Scholar]
- Fain J. N., García-Sáinz J. A. Role of phosphatidylinositol turnover in alpha 1 and of adenylate cyclase inhibition in alpha 2 effects of catecholamines. Life Sci. 1980 Apr 14;26(15):1183–1194. doi: 10.1016/0024-3205(80)90062-4. [DOI] [PubMed] [Google Scholar]
- Goldberg N. D., Haddox M. K. Cyclic GMP metabolism and involvement in biological regulation. Annu Rev Biochem. 1977;46:823–896. doi: 10.1146/annurev.bi.46.070177.004135. [DOI] [PubMed] [Google Scholar]
- Kimura H., Murad F. Two forms of guanylate cyclase in mammalian tissues and possible mechanisms for their regulation. Metabolism. 1975 Mar;24(3):439–445. doi: 10.1016/0026-0495(75)90123-7. [DOI] [PubMed] [Google Scholar]
- Lamerton L. F., Steel G. G. Growth kinetics of human large bowel cancer growing in immune-deprived mice and some chemotherapeutic observations. Cancer. 1975 Dec;36(6 Suppl):2431–2436. doi: 10.1002/1097-0142(197512)36:6<2431::aid-cncr2820360625>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Lands A. M., Arnold A., McAuliff J. P., Luduena F. P., Brown T. G., Jr Differentiation of receptor systems activated by sympathomimetic amines. Nature. 1967 May 6;214(5088):597–598. doi: 10.1038/214597a0. [DOI] [PubMed] [Google Scholar]
- Nowak K., Peckham M. J., Steel G. G. Variation in response of xenografts of colo-rectal carcinoma to chemotherapy. Br J Cancer. 1978 Apr;37(4):576–584. doi: 10.1038/bjc.1978.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlmutter A. F., Rapino E., Saffran M. ACTH and cyclic adenine nucleotides do not provoke identical adrenocortical responses. Endocrinology. 1971 Oct;89(4):963–968. doi: 10.1210/endo-89-4-963. [DOI] [PubMed] [Google Scholar]
- Samir Amer M., Kreighbaum W. E. Cyclic nucleotide phosphodiesterases: properties, activators, inhibitors, structure--activity relationships, and possible role in drug development. J Pharm Sci. 1975 Jan;64(1):1–37. doi: 10.1002/jps.2600640106. [DOI] [PubMed] [Google Scholar]
- Steel G. G., Courtenay V. D., Rostom A. Y. Improved immune-suppression techniques for the exongrafting of human tumours. Br J Cancer. 1978 Feb;37(2):224–230. doi: 10.1038/bjc.1978.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutton P. J., Barkla D. H. Cell proliferation in the descending colon of dimethylhydrazine treated rats and in dimethylhydrazine induced adenocarcinomata. Virchows Arch B Cell Pathol. 1976 Aug 11;21(2):147–160. doi: 10.1007/BF02899151. [DOI] [PubMed] [Google Scholar]
- Tutton P. J., Barkla D. H. Cytotoxicity of 5,6-dihydroxytryptamine in dimethylhydrazine-induced carcinomas of rat colon. Cancer Res. 1977 Apr;37(4):1241–1244. [PubMed] [Google Scholar]
- Tutton P. J., Barkla D. H. Evaluation of the cytotoxicity of dihydroxytryptamines and 5-hydroxytryptamine antagonists as cytotoxic agents in dimethylhydrazine-induced adenocarcinomata. Cancer Chemother Pharmacol. 1978;1(4):209–213. doi: 10.1007/BF00257151. [DOI] [PubMed] [Google Scholar]
- Tutton P. J., Barkla D. H. Neural control of colonic cell proliferation. Cancer. 1980 Mar 15;45(5 Suppl):1172–1177. doi: 10.1002/1097-0142(19800315)45:5+<1172::aid-cncr2820451322>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Tutton P. J., Barkla D. H. Stimulation of cell proliferation by histamine H2 receptors in dimethylhdrazine-induced adenocarcinomata. Cell Biol Int Rep. 1978 Mar;2(2):199–202. doi: 10.1016/0309-1651(78)90043-7. [DOI] [PubMed] [Google Scholar]
- Tutton P. J., Barkla D. H. The influence of adrenoceptor activity on cell proliferation in colonic crypt ipithelium and in colonic adenocarcinomata. Virchows Arch B Cell Pathol. 1977 Jun 24;24(2):139–146. doi: 10.1007/BF02889274. [DOI] [PubMed] [Google Scholar]
- Tutton P. J., Barkla D. H. The influence of dibutyryl adenosine cyclic monophosphate on cell proliferation in the epithelium of the jejunal crypts, the colonic crypts and in colonic carcinomata of rat. Clin Exp Pharmacol Physiol. 1980 May-Jun;7(3):275–280. doi: 10.1111/j.1440-1681.1980.tb00071.x. [DOI] [PubMed] [Google Scholar]
- Tutton P. J., Barkla D. H. The influence of serotonin on the mitotic rate in the colonic crypt epithelium and in colonic adenocarcinoma in rats. Clin Exp Pharmacol Physiol. 1978 Jan-Feb;5(1):91–94. doi: 10.1111/j.1440-1681.1978.tb00657.x. [DOI] [PubMed] [Google Scholar]
- Tutton P. J., Steel G. G. Influence of biogenic amines on the growth of xenografted human colorectal carcinomas. Br J Cancer. 1979 Nov;40(5):743–749. doi: 10.1038/bjc.1979.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutton P. J. The influence of thyroidectomy and of triiodothyronine administration on epithelial cell proliferation in the jejunum of rat. Virchows Arch B Cell Pathol. 1976 Mar 9;20(2):139–142. doi: 10.1007/BF02890334. [DOI] [PubMed] [Google Scholar]


