Abstract
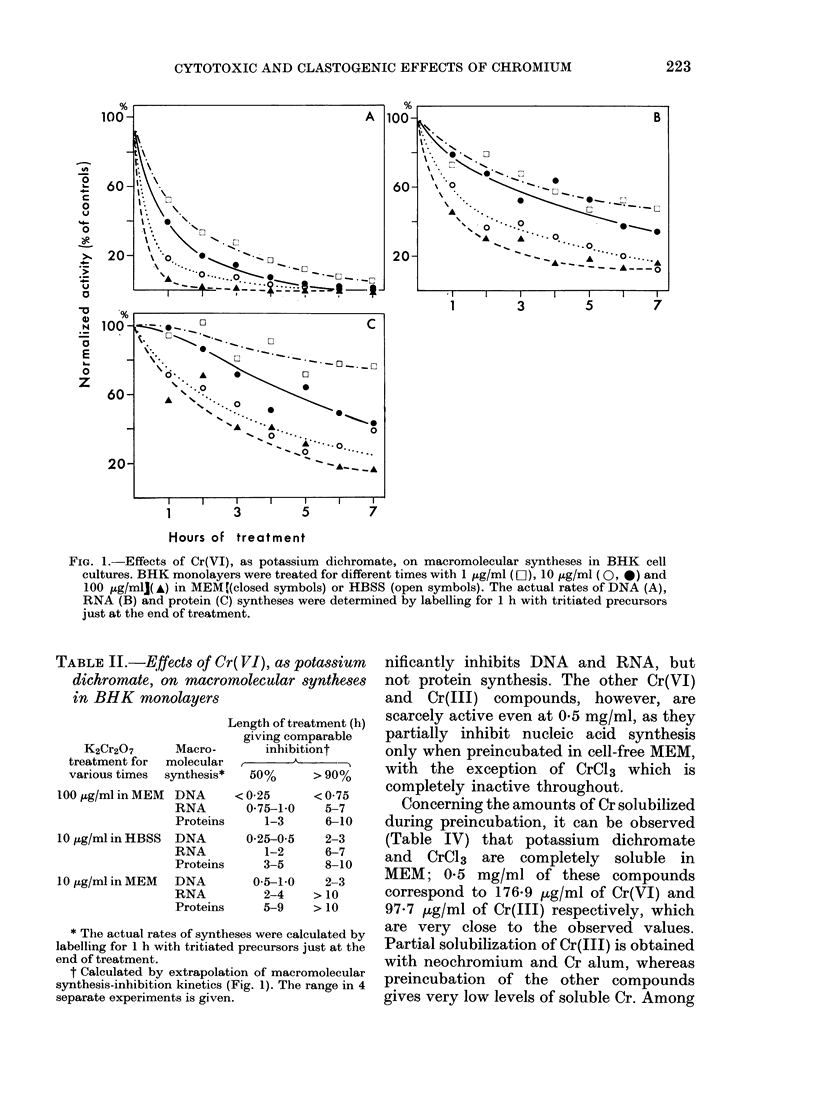
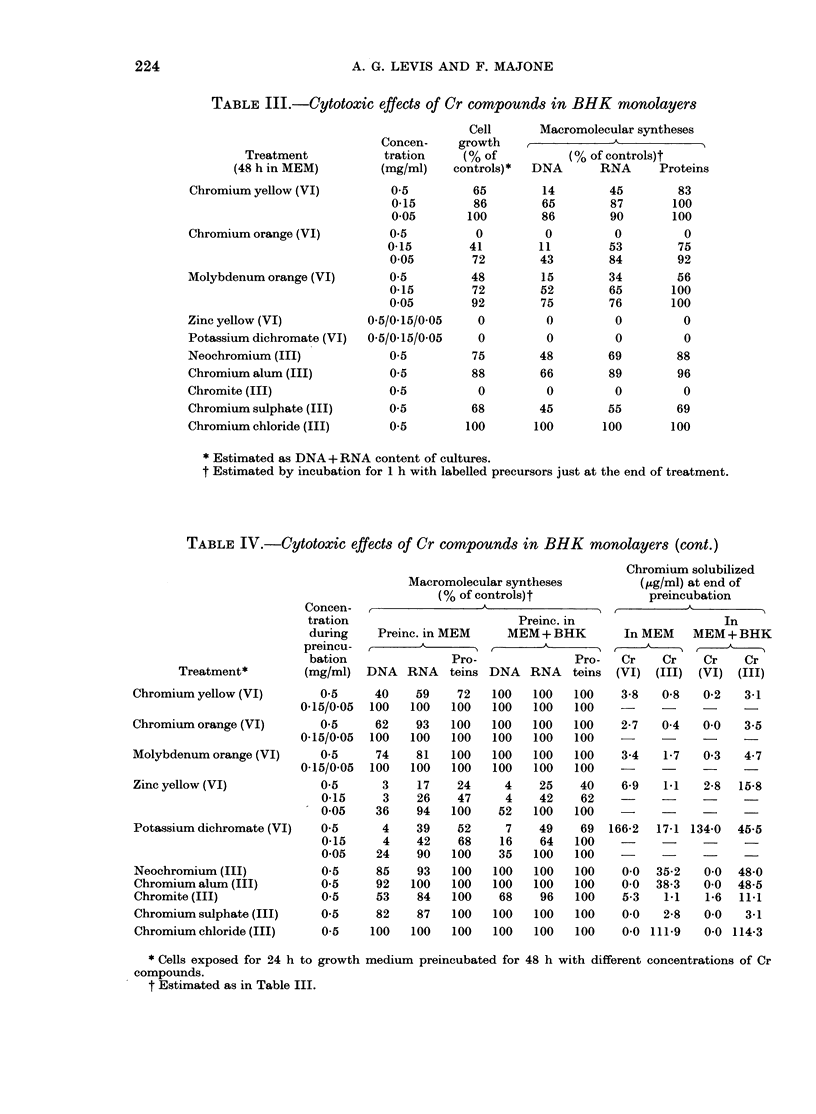
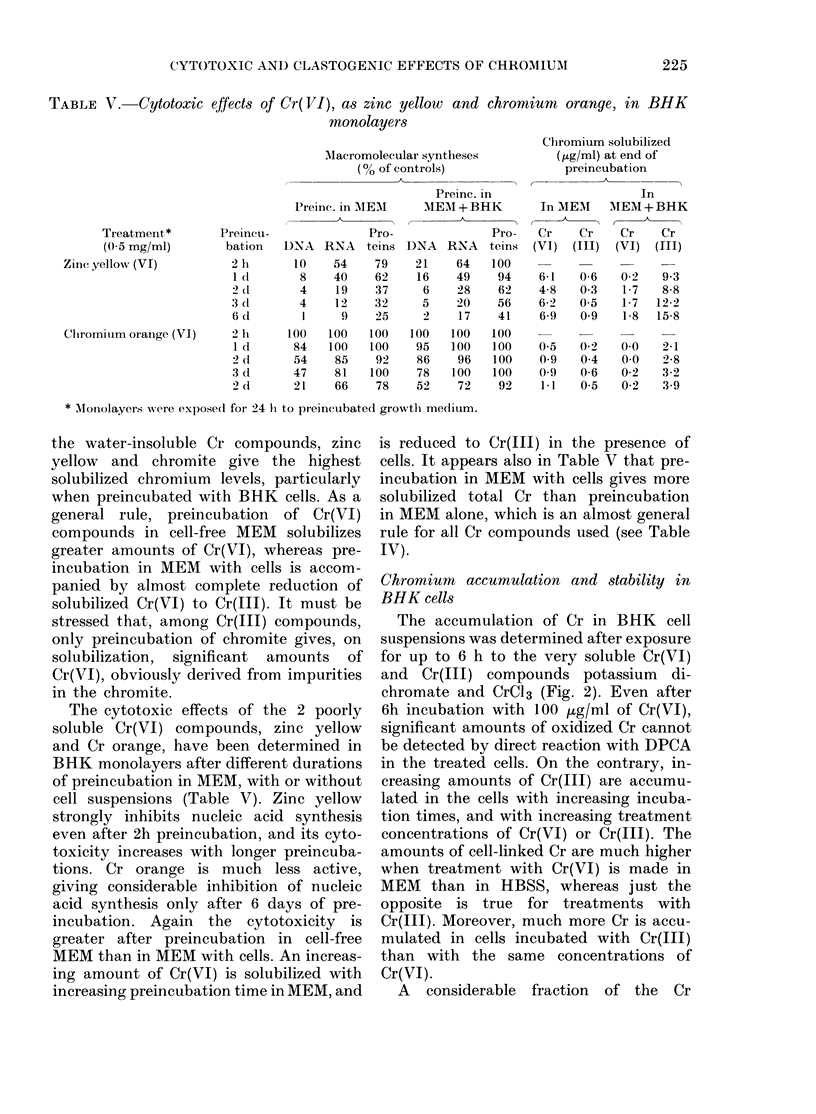
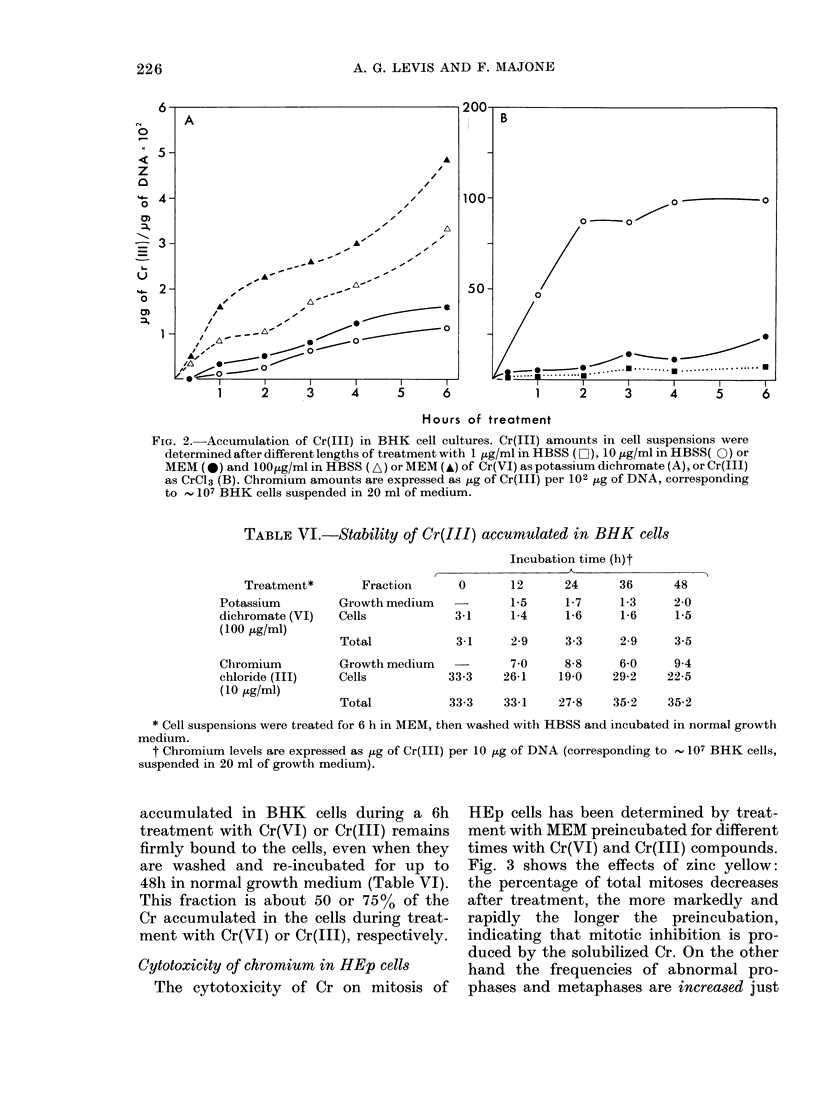
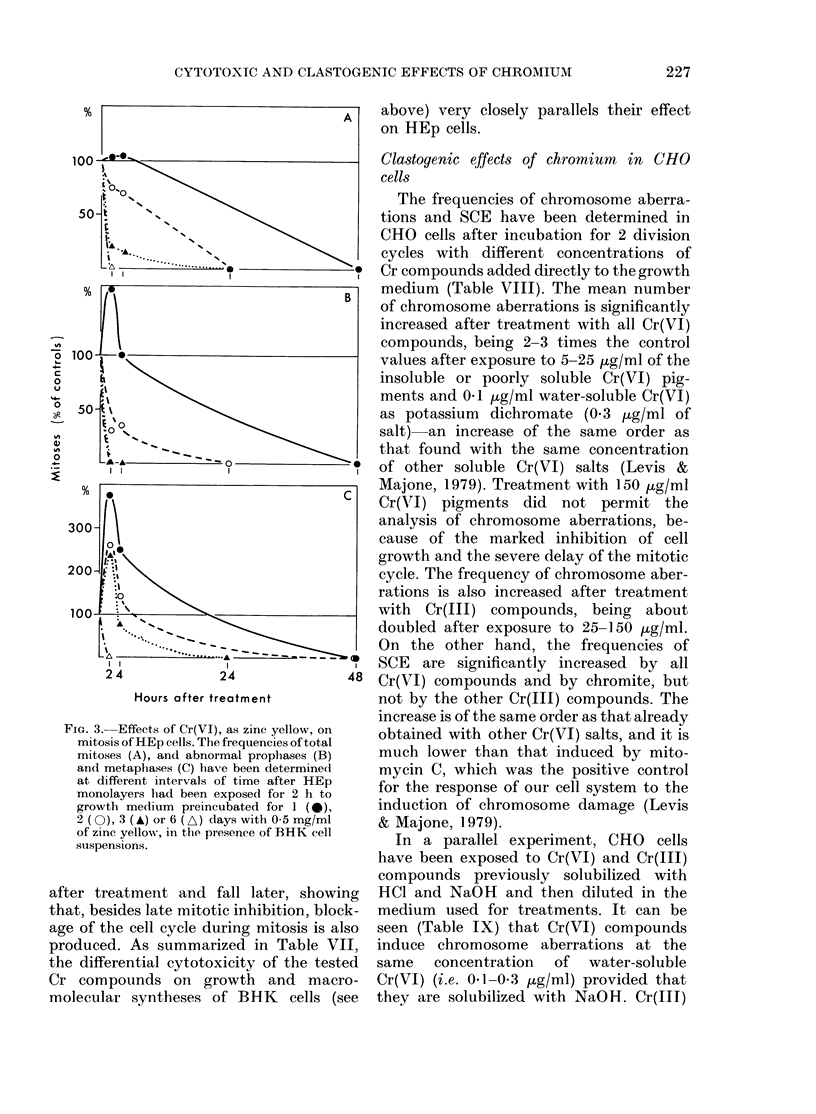
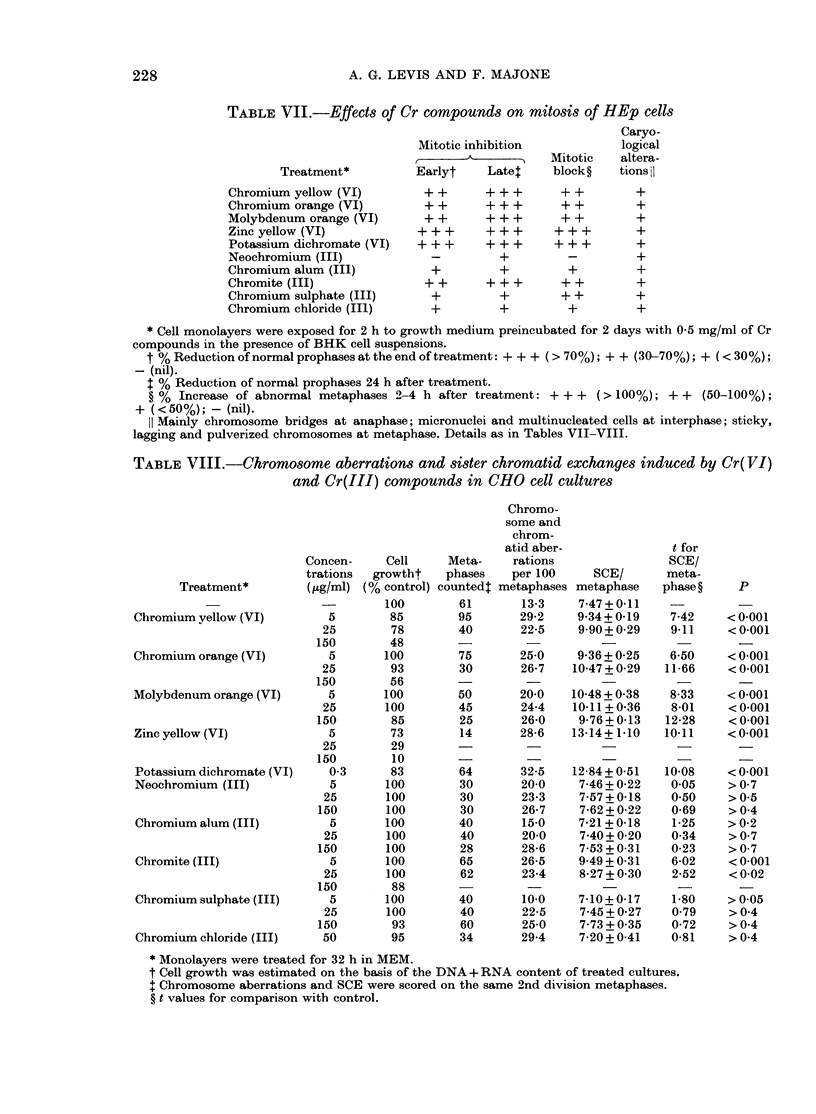
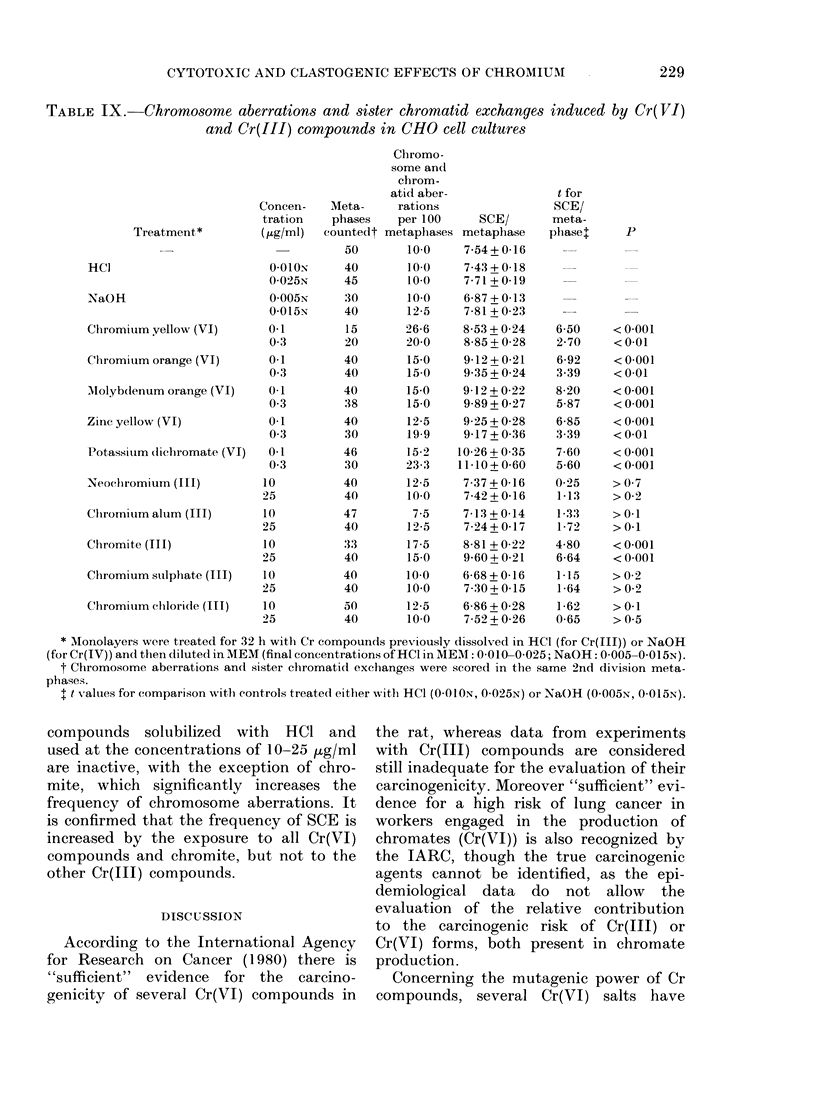
Cr(III) and Cr(VI) compounds of varying solubilities have been tested in vitro for their ability to inhibit cell growth and nucleic acid and protein syntheses in BHK cells, to induce alterations in the mitotic cycle in HEp cells, and to increase the frequency of chromosomal aberrations and sister chromatid exchanges (SCE) in CHO cells. All Cr(VI) compounds, and particularly those containing soluble Cr(VI), such as potassium dichromate and zinc yellow, differentially inhibit macromolecular syntheses in BKH cells, that of DNA being always the most affected. Among Cr(III) compounds, which generally have very low cytotoxicity, chromite is particularly active, and inhibits cell growth and DNA synthesis even more than the poorly soluble Cr(VI) compounds. Preincubation in growth medium, with or without metabolizing cell cultures, solubilizes considerable amounts of Cr(VI) from zinc yellow and chromite, but significant amounts are also obtained from the most insoluble Cr(VI) pigments. When BHK cells are treated with such preincubated solutions, reduction of soluble Cr(VI) to Cr(III) by cell metabolites is seen with all Cr(VI) compounds, accompanied by decreased cytotoxicity. The same differences between Cr(VI) and Cr(III) compounds apply to the cytotoxic effects on mitosis of HEp cells and the clastogenic effects on CHO cells. The activity of chromite, the only Cr(III) pigment capable of significantly increasing the frequency of SCE, is due to contamination with soluble Cr(VI). In contrast to the very low cytotoxicity of Cr(III), much higher chromium levels are detected in the cells incubated with soluble Cr(III) than with the same concentrations of soluble Cr(VI). 50% and 75% of chromium accumulated in the cells during treatments with Cr(VI) and Cr(III) respectively remains firmly bound to the cells, even when they are incubated for up to 48 h in normal growth medium. Chromium accumulated in the cells after treatment with Cr(III) is most probably bound to the cell membrane, whereas some of the Cr(VI) is transported through the cell membrane and reduced in the cell nucleus. The results of the present investigation are in agreement with those obtained with the same Cr(VI) and Cr(III) compounds in mutagenicity assays in bacteria and carcinogenicity tests in rodents. A re-evaluation of the mechanisms of chromium carcinogenisis is proposed.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bianchi V., Dal Toso R., Debetto P., Levis A. G., Luciani S., Majone F., Tamino G. Mechanisms of chromium toxicity in mammalian cell cultures. Toxicology. 1980;17(2):219–224. doi: 10.1016/0300-483x(80)90097-9. [DOI] [PubMed] [Google Scholar]
- Bianchi V., Levis A. G., Saggioro D. Differential cytotoxic activity of potassium dichromate on nucleoside uptake in BHK fibroblasts. Chem Biol Interact. 1979 Feb;24(2):137–151. doi: 10.1016/0009-2797(79)90003-6. [DOI] [PubMed] [Google Scholar]
- Bonatti S., Meini M., Abbondandolo A. Genetic effects of potassium dichromate in Schizosaccharomyces pombe. Mutat Res. 1976 Apr;38(2):147–150. [PubMed] [Google Scholar]
- Casto B. C., Meyers J., DiPaolo J. A. Enhancement of viral transformation for evaluation of the carcinogenic or mutagenic potential of inorganic metal salts. Cancer Res. 1979 Jan;39(1):193–198. [PubMed] [Google Scholar]
- De Flora S., Boido V. Effect of human gastric juice on the mutagenicity of chemicals. Mutat Res. 1980 Apr;77(4):307–315. doi: 10.1016/0165-1218(80)90002-6. [DOI] [PubMed] [Google Scholar]
- De Flora S. Metabolic deactivation of mutagens in the Salmonella-microsome test. Nature. 1978 Feb 2;271(5644):455–456. doi: 10.1038/271455a0. [DOI] [PubMed] [Google Scholar]
- De Flora S. Study of 106 organic and inorganic compounds in the Salmonella/microsome test. Carcinogenesis. 1981;2(4):283–298. doi: 10.1093/carcin/2.4.283. [DOI] [PubMed] [Google Scholar]
- DiPaolo J. A., Casto B. C. Quantitative studies of in vitro morphological transformation of Syrian hamster cells by inorganic metal salts. Cancer Res. 1979 Mar;39(3):1008–1013. [PubMed] [Google Scholar]
- Douglas G. R., Bell R. D., Grant C. E., Wytsma J. M., Bora K. C. Effect of lead chromate on chromosome aberration, sister-chromatid exchange and DNA damage in mammalian cells in vitro. Mutat Res. 1980 Feb;77(2):157–163. doi: 10.1016/0165-1218(80)90133-0. [DOI] [PubMed] [Google Scholar]
- Fradkin A., Janoff A., Lane B. P., Kuschner M. In vitro transformation of BHK21 cells grown in the presence of calcium chromate. Cancer Res. 1975 Apr;35(4):1058–1063. [PubMed] [Google Scholar]
- GROGAN C. H. Experimental studies in metal cancerigenesis. VIII. On the etiological factor in chromate cancer. Cancer. 1957 May-Jun;10(3):625–638. doi: 10.1002/1097-0142(195705/06)10:3<625::aid-cncr2820100330>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Green M. H., Muriel W. J., Bridges B. A. Use of a simplified fluctuation test to detect low levels of mutagens. Mutat Res. 1976 Feb;38(1):33–42. doi: 10.1016/0165-1161(76)90077-7. [DOI] [PubMed] [Google Scholar]
- Kanematsu N., Hara M., Kada T. Rec assay and mutagenicity studies on metal compounds. Mutat Res. 1980 Feb;77(2):109–116. doi: 10.1016/0165-1218(80)90127-5. [DOI] [PubMed] [Google Scholar]
- Knudsen I. The mammalian spot test and its use for the testing of potential carcinogenicity of welding fume particles and hexavalent chromium. Acta Pharmacol Toxicol (Copenh) 1980 Jul;47(1):66–70. doi: 10.1111/j.1600-0773.1980.tb02027.x. [DOI] [PubMed] [Google Scholar]
- Levis A. G., Bianchi V., Tamino G., Pegoraro B. Cytotoxic effects of hexavalent and trivalent chromium on mammalian cells in vitro. Br J Cancer. 1978 Mar;37(3):386–396. doi: 10.1038/bjc.1978.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis A. G., Buttignol M., Bianchi V., Sponza G. Effects of potassium dichromate on nucleic acid and protein syntheses and on precursor uptake in BHK fibroblasts. Cancer Res. 1978 Jan;38(1):110–116. [PubMed] [Google Scholar]
- Levis A. G., Majone F. Cytotoxic and clastogenic effects of soluble chromium compounds on mammalian cell cultures. Br J Cancer. 1979 Oct;40(4):523–533. doi: 10.1038/bjc.1979.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciani S., Dal Toso R., Rebellato A. M., Levis A. G. Effects of chromium compounds on plasma membrane Mg2+-ATPase activity of BHK cells. Chem Biol Interact. 1979 Sep;27(1):59–67. doi: 10.1016/0009-2797(79)90149-2. [DOI] [PubMed] [Google Scholar]
- Léonard A., Lauwerys R. R. Carcinogenicity and mutagenicity of chromium. Mutat Res. 1980 Nov;76(3):227–239. doi: 10.1016/0165-1110(80)90018-4. [DOI] [PubMed] [Google Scholar]
- MacRae W. D., Whiting R. F., Stich H. F. Sister chromatid exchanges induced in cultured mammalian cells by chromate. Chem Biol Interact. 1979 Aug;26(3):281–286. doi: 10.1016/0009-2797(79)90031-0. [DOI] [PubMed] [Google Scholar]
- Majone F., Levis A. G. Chromosomal aberrations and sister-chromatid exchanges in Chinese hamster cells treated in vitro with hexavalent chromium compounds. Mutat Res. 1979 Jul;67(3):231–238. doi: 10.1016/0165-1218(79)90017-x. [DOI] [PubMed] [Google Scholar]
- Maltoni C. Occupational carcinogenesis. Predictive value of carcinogenesis bioassays. Ann N Y Acad Sci. 1976;271:431–443. doi: 10.1111/j.1749-6632.1976.tb23144.x. [DOI] [PubMed] [Google Scholar]
- Mertz W. Chromium occurrence and function in biological systems. Physiol Rev. 1969 Apr;49(2):163–239. doi: 10.1152/physrev.1969.49.2.163. [DOI] [PubMed] [Google Scholar]
- Nakamuro K., Yoshikawa K., Sayato Y., Kurata H. Comparative studies of chromosomal aberration and mutagenicity of the trivalent and hexavalent chromium. Mutat Res. 1978 Nov;58(2-3):175–181. doi: 10.1016/0165-1218(78)90007-1. [DOI] [PubMed] [Google Scholar]
- Nestmann E. R., Matula T. I., Douglas G. R., Bora K. C., Kowbel D. J. Detection of the mutagenic activity of lead chromate using a battery of microbial tests. Mutat Res. 1979 Apr;66(4):357–365. doi: 10.1016/0165-1218(79)90046-6. [DOI] [PubMed] [Google Scholar]
- Newbold R. F., Amos J., Connell J. R. The cytotoxic, mutagenic and clastogenic effects of chromium-containing compounds on mammalian cells in culture. Mutat Res. 1979 May;67(1):55–63. doi: 10.1016/0165-1218(79)90099-5. [DOI] [PubMed] [Google Scholar]
- Nishioka H. Mutagenic activities of metal compounds in bacteria. Mutat Res. 1975 Jun;31(3):185–189. doi: 10.1016/0165-1161(75)90088-6. [DOI] [PubMed] [Google Scholar]
- Petrilli F. L., De Flora S. Metabolic deactivation of hexavalent chromium mutagenicity. Mutat Res. 1978 Oct;54(2):139–147. doi: 10.1016/0165-1161(78)90034-1. [DOI] [PubMed] [Google Scholar]
- Petrilli F. L., de Flora S. Oxidation of inactive trivalent chromium to the mutagenic hexavalent form. Mutat Res. 1978 Nov;58(2-3):167–173. doi: 10.1016/0165-1218(78)90006-x. [DOI] [PubMed] [Google Scholar]
- Savory J., Mushak P., Sunderman F. W., Jr, Estes R. H., Roszel N. O. Microdetermination of chromium in biological materials by gas chromatography. Anal Chem. 1970 Feb;42(2):294–297. doi: 10.1021/ac60284a039. [DOI] [PubMed] [Google Scholar]
- Sirover M. A., Loeb L. A. Infidelity of DNA synthesis in vitro: screening for potential metal mutagens or carcinogens. Science. 1976 Dec 24;194(4272):1434–1436. doi: 10.1126/science.1006310. [DOI] [PubMed] [Google Scholar]
- Tkeshelashvili L. K., Shearman C. W., Zakour R. A., Koplitz R. M., Loeb L. A. Effects of arsenic, selenium, and chromium on the fidelity of DNA synthesis. Cancer Res. 1980 Jul;40(7):2455–2460. [PubMed] [Google Scholar]
- Tsuda H., Kato K. Chromosomal aberrations and morphological transformation in hamster embryonic cells treated with potassium dichromate in vitro. Mutat Res. 1977 Apr;46(2):87–94. doi: 10.1016/0165-1161(77)90115-7. [DOI] [PubMed] [Google Scholar]
- Umeda M., Nishimura M. Inducibility of chromosomal aberrations by metal compounds in cultured mammalian cells. Mutat Res. 1979 Jul;67(3):221–229. doi: 10.1016/0165-1218(79)90016-8. [DOI] [PubMed] [Google Scholar]
- Vallée M. Le système de transport de sulfate chez Chlorella pyrenoidosaa et sa régulation. IV. Etudes avec l'ion chromate. Biochim Biophys Acta. 1969 Apr;173(3):486–500. doi: 10.1016/0005-2736(69)90013-3. [DOI] [PubMed] [Google Scholar]
- Venitt S., Levy L. S. Mutagenicity of chromates in bacteria and its relevance to chromate carcinogenesis. Nature. 1974 Aug 9;250(5466):493–495. doi: 10.1038/250493a0. [DOI] [PubMed] [Google Scholar]
- White L. R., Jakobsen K., Ostgaard K. Comparative toxicity studies of chromium-rich welding fumes and chromium on an established human cell line. Environ Res. 1979 Dec;20(2):366–374. doi: 10.1016/0013-9351(79)90013-6. [DOI] [PubMed] [Google Scholar]
- Whiting R. F., Stich H. F., Koropatnick D. J. DNA damage and DNA repair in cultured human cells exposed to chromate. Chem Biol Interact. 1979 Aug;26(3):267–280. doi: 10.1016/0009-2797(79)90030-9. [DOI] [PubMed] [Google Scholar]
- Wild D. Cytogenetic effects in the mouse of 17 chemical mutagens and carcinogens evaluated by the micronucleus test. Mutat Res. 1978 Jan;56(3):319–327. doi: 10.1016/0027-5107(78)90200-2. [DOI] [PubMed] [Google Scholar]


