Abstract
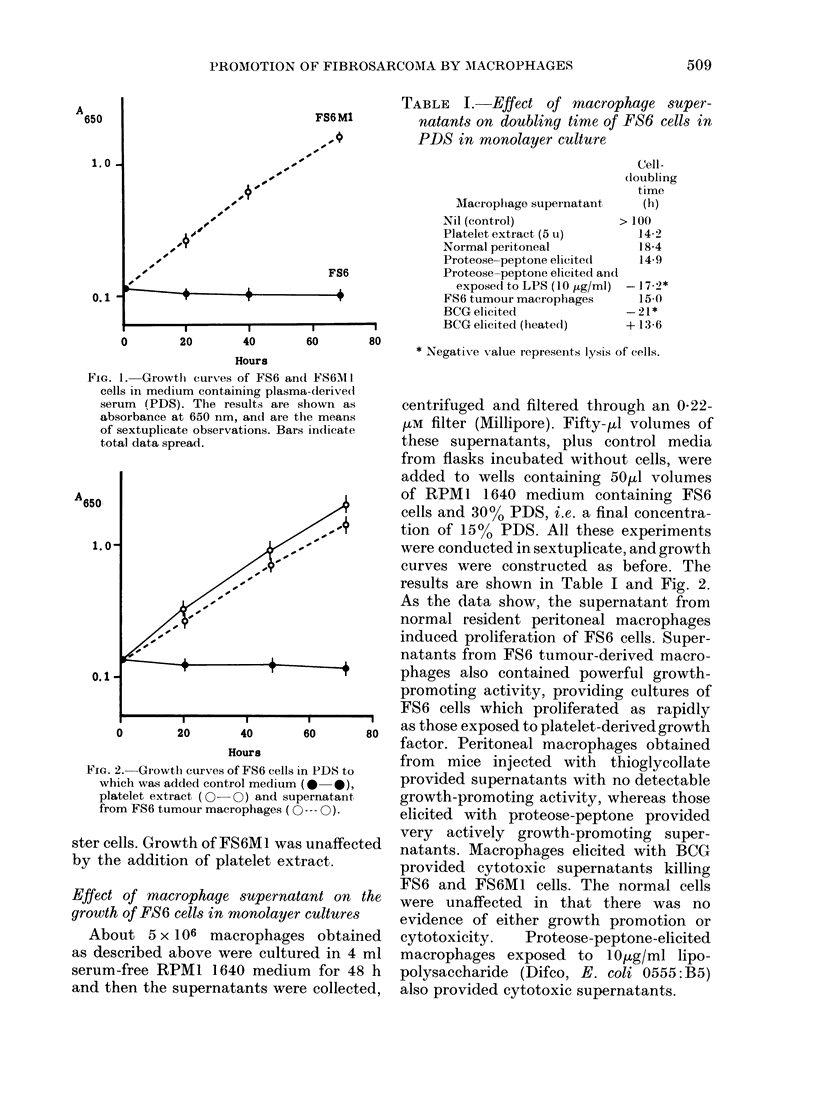
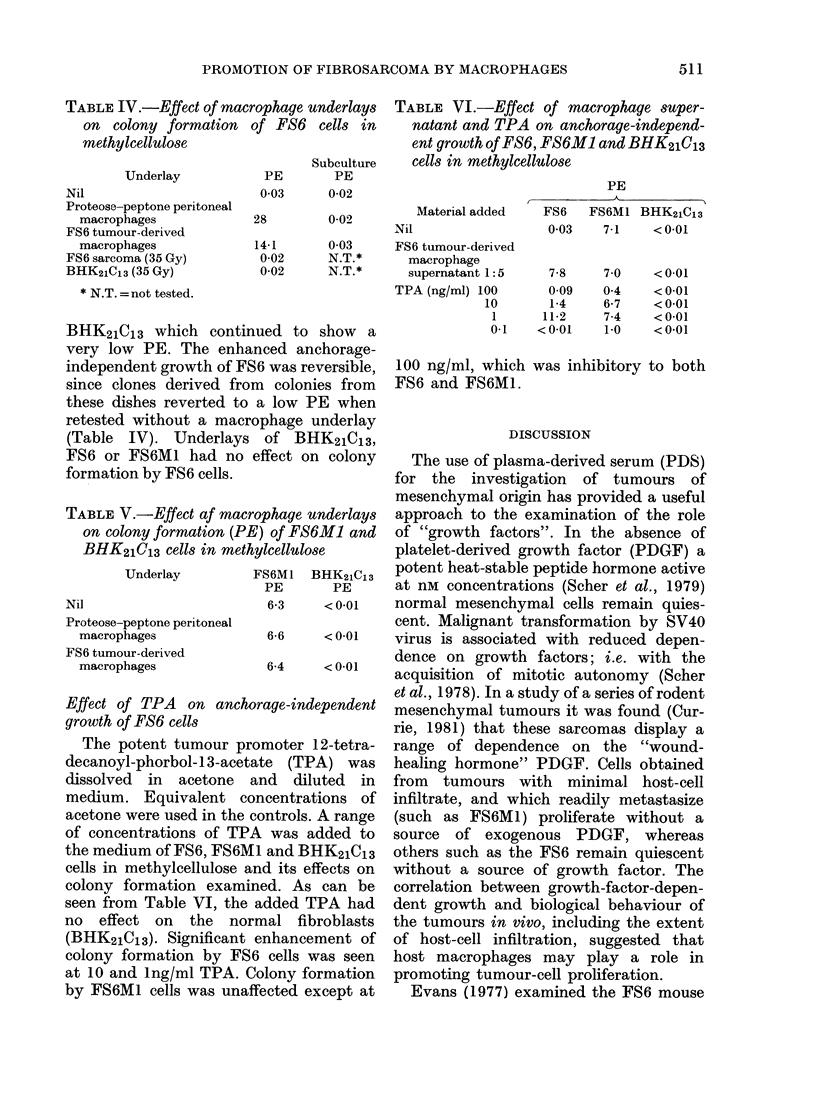
Cells from a C57BL/cbi chemically induced fibrosarcoma (FS6) require exogenous platelet-derived growth factor (PDGF) for in vitro proliferation (as do normal "untransformed" fibroblasts) whereas cells obtained from the FS6M1 tumour, a spontaneous metastasizing subline, show autonomy from PDGF in vitro. Furthermore, the FS6 cells exhibit very low colony formation in an anchorage-independent growth assay. In vivo, this tumour is immunogenic, rarely metastasizes and is heavily infiltrated by host macrophages. Studies of in vitro cell proliferation and anchorage-independent growth show that syngeneic host macrophages from the peritoneal cavity or from the growing tumour release a diffusible factor(s) which has (1) growth-stimulating activity on FS6 cells in monolayer cultures in PDGF-poor medium and (2) potent colony-stimulating activity on FS6 cell cultured in methyl-cellulose-containing medium. These macrophage supernatants stimulate proliferation of quiescent normal fibroblasts in monolayer culture as well as FS6 sarcoma cells, but do not stimulate anchorage-independent growth of normal cells. Supernatants from BCG-elicited macrophages were shown to contain abundant arginase, and were cytolytic to FS6 cells but not to normal cells. Heat inactivation abrogated the arginase and cytotoxicity, revealing heat-stable mitogenicity for FS6 cells and normal fibroblasts. The stimulatory effect of macrophages on FS6 sarcoma cells can be mimicked by the addition of the tumour promoter 12-tetradecanoyl-phorbol-13-acetate (TPA) and supports the hypothesis that macrophages could play a significant role in multistage carcinogenesis by providing a source of endogenous promoter.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Currie G. A. Activated macrophages kill tumour cells by releasing arginase. Nature. 1978 Jun 29;273(5665):758–759. doi: 10.1038/273758a0. [DOI] [PubMed] [Google Scholar]
- Currie G. A., Basham C. Differential arginine dependence and the selective cytotoxic effects of activated macrophages for malignant cells in vitro. Br J Cancer. 1978 Dec;38(6):653–659. doi: 10.1038/bjc.1978.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie G. A. Platelet-derived growth-factor requirements for in vitro proliferation of normal and malignant mesenchymal cells. Br J Cancer. 1981 Mar;43(3):335–343. doi: 10.1038/bjc.1981.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLustro F., Sherer G. K., LeRoy E. C. Human monocyte stimulation of fibroblast growth by a soluble mediator(s). J Reticuloendothel Soc. 1980 Dec;28(6):519–532. [PubMed] [Google Scholar]
- Evans R. Effect of X-irradiation on host-cell infiltration and growth of a murine fibrosarcoma. Br J Cancer. 1977 May;35(5):557–566. doi: 10.1038/bjc.1977.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. Host cells in transplanted murine tumors and their possible relevance to tumor growth. J Reticuloendothel Soc. 1979 Oct;26(4):427–437. [PubMed] [Google Scholar]
- Filkins J. P. Endotoxin-enhanced secretion of macrophage insulin-like activity. J Reticuloendothel Soc. 1980 May;27(5):507–511. [PubMed] [Google Scholar]
- Greenburg G. B., Hunt T. K. The proliferative response in vitro of vascular endothelial and smooth muscle cells exposed to wound fluids and macrophages. J Cell Physiol. 1978 Dec;97(3 Pt 1):353–360. doi: 10.1002/jcp.1040970310. [DOI] [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. A macrophage-dependent factor that stimulates the proliferation of fibroblasts in vitro. Am J Pathol. 1976 Sep;84(3):501–514. [PMC free article] [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975 Jan;78(1):71–100. [PMC free article] [PubMed] [Google Scholar]
- Mantovani A. Effects on in vitro tumor growth of murine macrophages isolated from sarcoma lines differing in immunogenicity and metastasizing capacity. Int J Cancer. 1978 Dec;22(6):741–746. doi: 10.1002/ijc.2910220617. [DOI] [PubMed] [Google Scholar]
- Polverini P. J., Cotran P. S., Gimbrone M. A., Jr, Unanue E. R. Activated macrophages induce vascular proliferation. Nature. 1977 Oct 27;269(5631):804–806. doi: 10.1038/269804a0. [DOI] [PubMed] [Google Scholar]
- Prehn R. T. Do tumors grow because of the immune response of the host? Transplant Rev. 1976;28:34–42. doi: 10.1111/j.1600-065x.1976.tb00191.x. [DOI] [PubMed] [Google Scholar]
- Salmon S. E., Hamburger A. W. Immunoproliferation and cancer: a common macrophage-derived promoter substance. Lancet. 1978 Jun 17;1(8077):1289–1290. doi: 10.1016/s0140-6736(78)91270-9. [DOI] [PubMed] [Google Scholar]
- Scher C. D., Pledger W. J., Martin P., Antoniades H., Stiles C. D. Transforming viruses directly reduce the cellular growth requirement for a platelet derived growth factor. J Cell Physiol. 1978 Dec;97(3 Pt 1):371–380. doi: 10.1002/jcp.1040970312. [DOI] [PubMed] [Google Scholar]
- Scher C. D., Shepard R. C., Antoniades H. N., Stiles C. D. Platelet-derived growth factor and the regulation of the mammalian fibroblast cell cycle. Biochim Biophys Acta. 1979 Aug 10;560(2):217–241. doi: 10.1016/0304-419x(79)90020-9. [DOI] [PubMed] [Google Scholar]


