Abstract
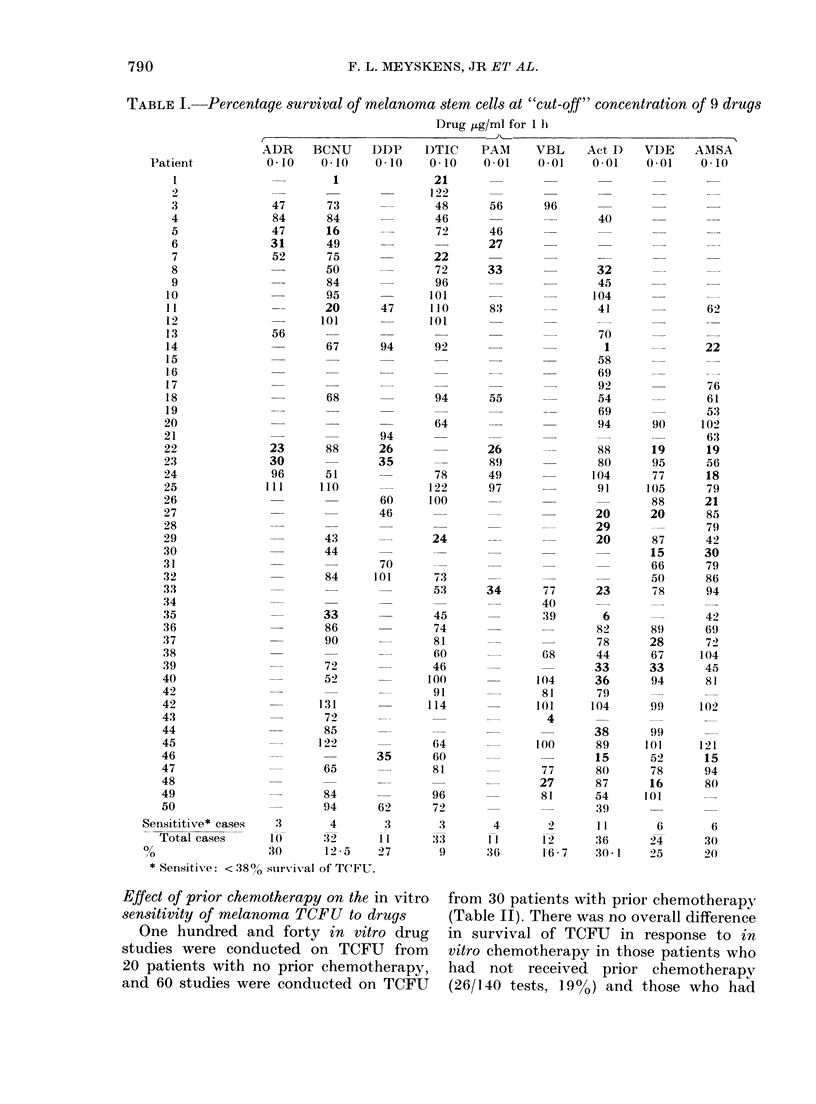
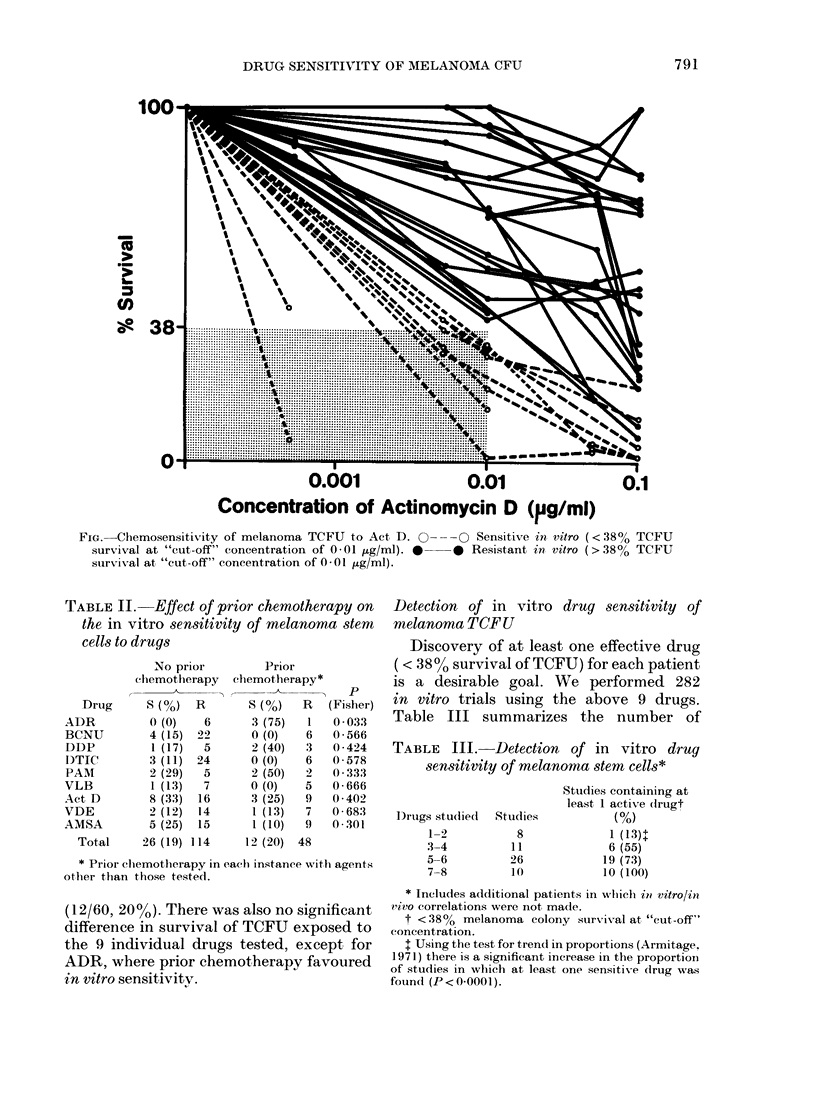
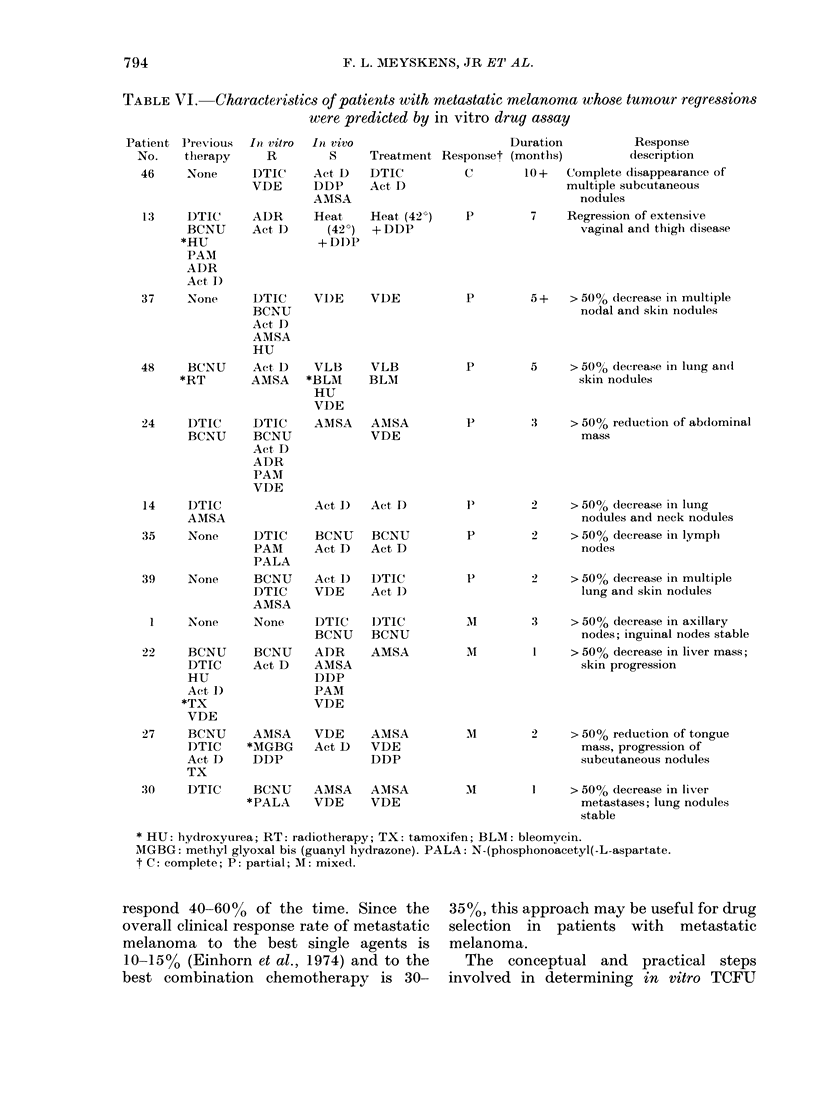
We measured the effect of 6 standard (Adriamycin, BCNU, DTIC, melphalan, vinblastine, actinomycin D) and 3 Phase II agents (cis-platinum, vindesine, AMSA) on melanoma colony-forming units (CFU) in soft agar from biopsies of 50 patients with metastatic melanoma. Melanoma CFU demonstrated marked heterogeneity in chemosensitivity to these 9 drugs. Reduction in survival of CFU below 38% at one-tenth the pharmacologically achievable 1h concentration (our operational definition of chemosensitivity) was obtained in only 19% of 200 in vitro trials, and was usually the same whether or not patients had been exposed to prior chemotherapy, suggesting that melanoma CFU are inherently resistant to presently available chemotherapeutic drugs. The soft-agar assay was 86% accurate (25/29 cases) in identifying drugs to which the tumour was resistant in vivo, and 63% accurate (12/19 trials) in identifying drugs to which the tumour was clinically sensitive, counting mixed responses as responses. In contrast, if mixed responses were classified as progressive disease, the accuracy of identification of sensitivity fell to 42% (8/19 trials). These investigations furnish a quantitative description of the chemosensitivity of human metastatic melanoma CFU. Additionally, these studies serve as a useful step towards the development of an in vitro chemosensitivity test for human melanoma, and provide an operational quantitative basis for further exploration of in vitro-directed therapy in metastatic neoplasms.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bateman A. E., Selby P. J., Steel G. G., Towse G. D. In vitro chemosensitivity tests on xenografted human melanomas. Br J Cancer. 1980 Feb;41(2):189–198. doi: 10.1038/bjc.1980.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtenay V. D., Mills J. An in vitro colony assay for human tumours grown in immune-suppressed mice and treated in vivo with cytotoxic agents. Br J Cancer. 1978 Feb;37(2):261–268. doi: 10.1038/bjc.1978.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einhorn L. H., Burgess M. A., Vallejos C., Bodey G. P., Sr, Gutterman J., Mavligit G., Hersh E. M., Luce J. K., Frei E., 3rd, Freireich E. J. Prognostic correlations and response to treatment in advanced metastatic malignant melanoma. Cancer Res. 1974 Aug;34(8):1995–2004. [PubMed] [Google Scholar]
- Fodstad O., Aass N., Pihl A. Response to chemotherapy of human, malignant melanoma xenografts in athymic, nude mice. Int J Cancer. 1980 Apr 15;25(4):453–458. doi: 10.1002/ijc.2910250405. [DOI] [PubMed] [Google Scholar]
- Hamburger A. W., Salmon S. E. Primary bioassay of human tumor stem cells. Science. 1977 Jul 29;197(4302):461–463. doi: 10.1126/science.560061. [DOI] [PubMed] [Google Scholar]
- Loo T. L., Housholder G. E., Gerulath A. H., Saunders P. H., Farquhar D. Mechanism of action and pharmacology studies with DTIC (NSC-45388). Cancer Treat Rep. 1976 Feb;60(2):149–152. [PubMed] [Google Scholar]
- MISHIMA Y. New technic for comprehensive demonstration of melanin, premelanin, and tyrosinase sites. Combined dopapremelanin reaction. J Invest Dermatol. 1960 Jun;34:355–360. doi: 10.1038/jid.1960.62. [DOI] [PubMed] [Google Scholar]
- Meyskens F. L., Jr, Soehnlen B. J., Saxe D. F., Casey W. J., Salmon S. E. In vitro clonal assay for human metastatic melanoma cells. Stem Cells. 1981;1(1):61–72. [PubMed] [Google Scholar]
- Salmon S. E., Buick R. N. Preparation of permanent slides of intact soft-agar colony cultures of hematopoietic and tumor stem cells. Cancer Res. 1979 Mar;39(3):1133–1136. [PubMed] [Google Scholar]
- Salmon S. E., Hamburger A. W., Soehnlen B., Durie B. G., Alberts D. S., Moon T. E. Quantitation of differential sensitivity of human-tumor stem cells to anticancer drugs. N Engl J Med. 1978 Jun 15;298(24):1321–1327. doi: 10.1056/NEJM197806152982401. [DOI] [PubMed] [Google Scholar]
- Salmon S. E., Meyskens F. L., Jr, Alberts D. S., Soehnlen B., Young L. New drugs in ovarian cancer and malignant melanoma: in vitro phase II screening with the human tumor stem cell assay. Cancer Treat Rep. 1981 Jan-Feb;65(1-2):1–12. [PubMed] [Google Scholar]
- Selby P. J., Steel G. G. Clonogenic cell survival in cryopreserved human tumour cells. Br J Cancer. 1981 Feb;43(2):143–148. doi: 10.1038/bjc.1981.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tveit K. M., Fodstad O., Olsnes S., Pihl A. In vitro sensitivity of human melanoma xenografts to cytotoxic drugs. Correlation with in vivo chemosensitivity. Int J Cancer. 1980 Dec 15;26(6):717–722. doi: 10.1002/ijc.2910260604. [DOI] [PubMed] [Google Scholar]
- Von Hoff D. D., Casper J., Bradley E., Sandbach J., Jones D., Makuch R. Association between human tumor colony-forming assay results and response of an individual patient's tumor to chemotherapy. Am J Med. 1981 May;70(5):1027–1041. doi: 10.1016/0002-9343(81)90859-7. [DOI] [PubMed] [Google Scholar]


