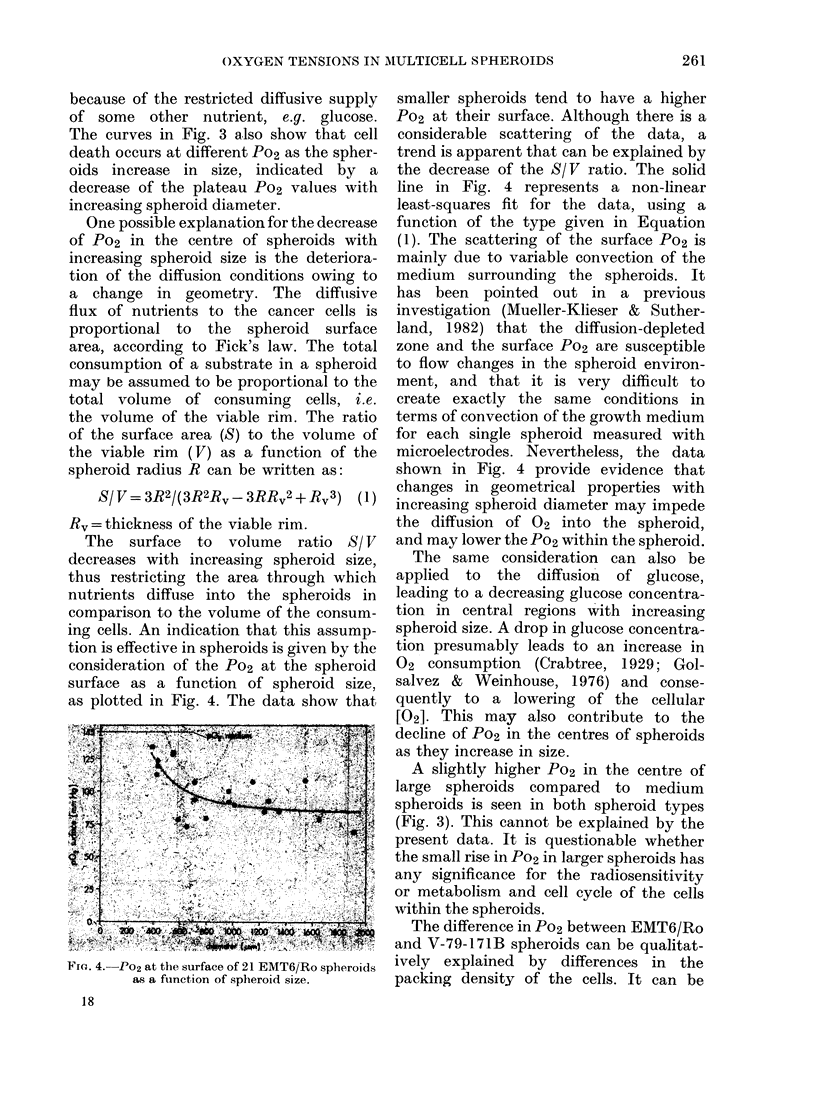
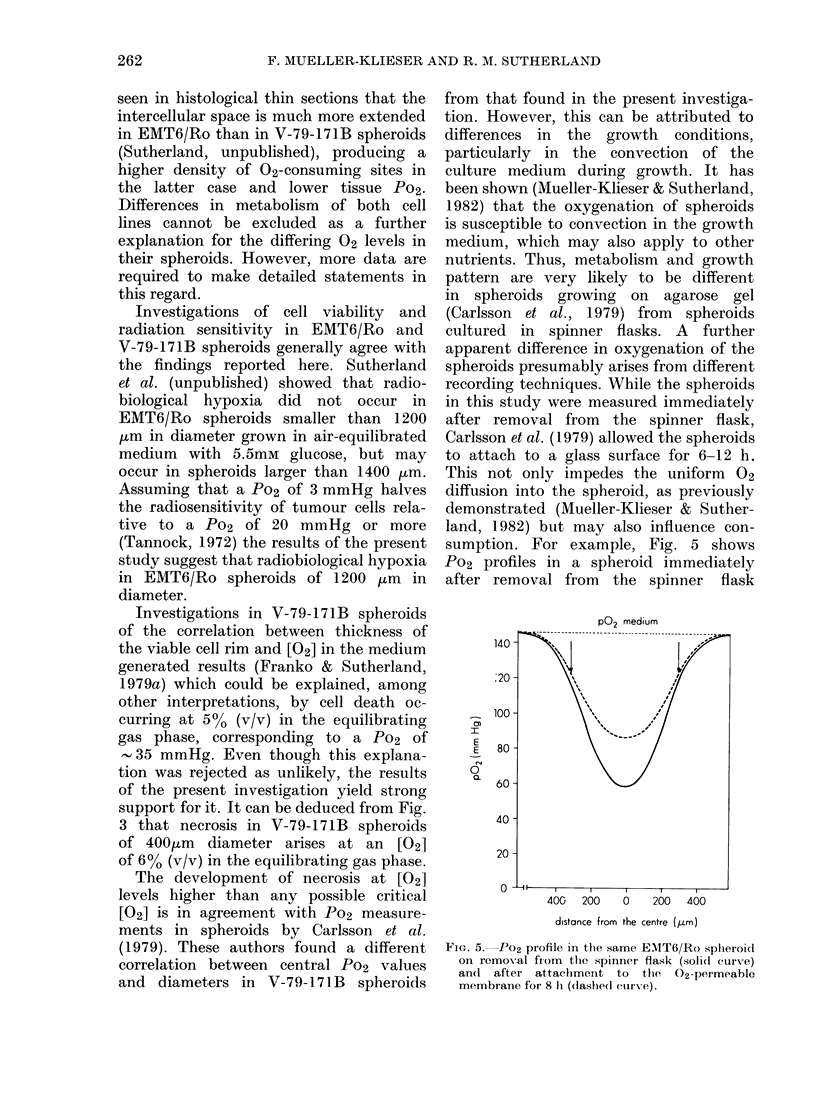
Abstract
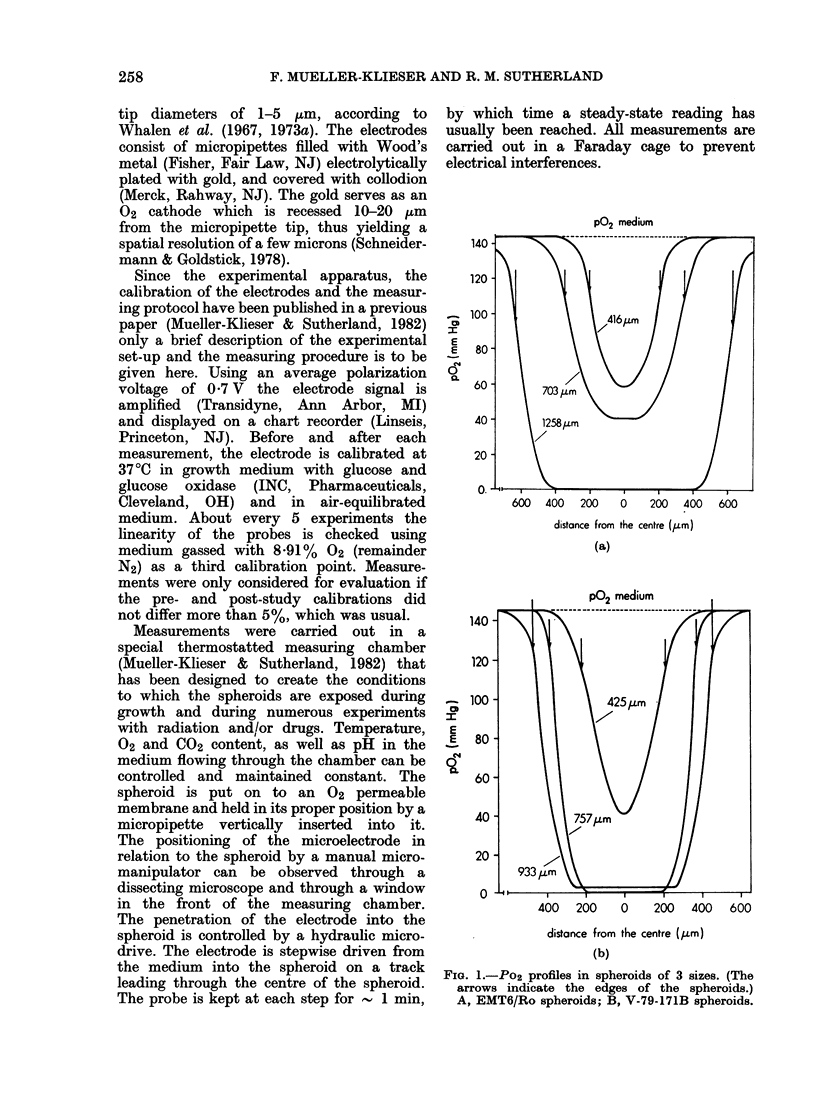
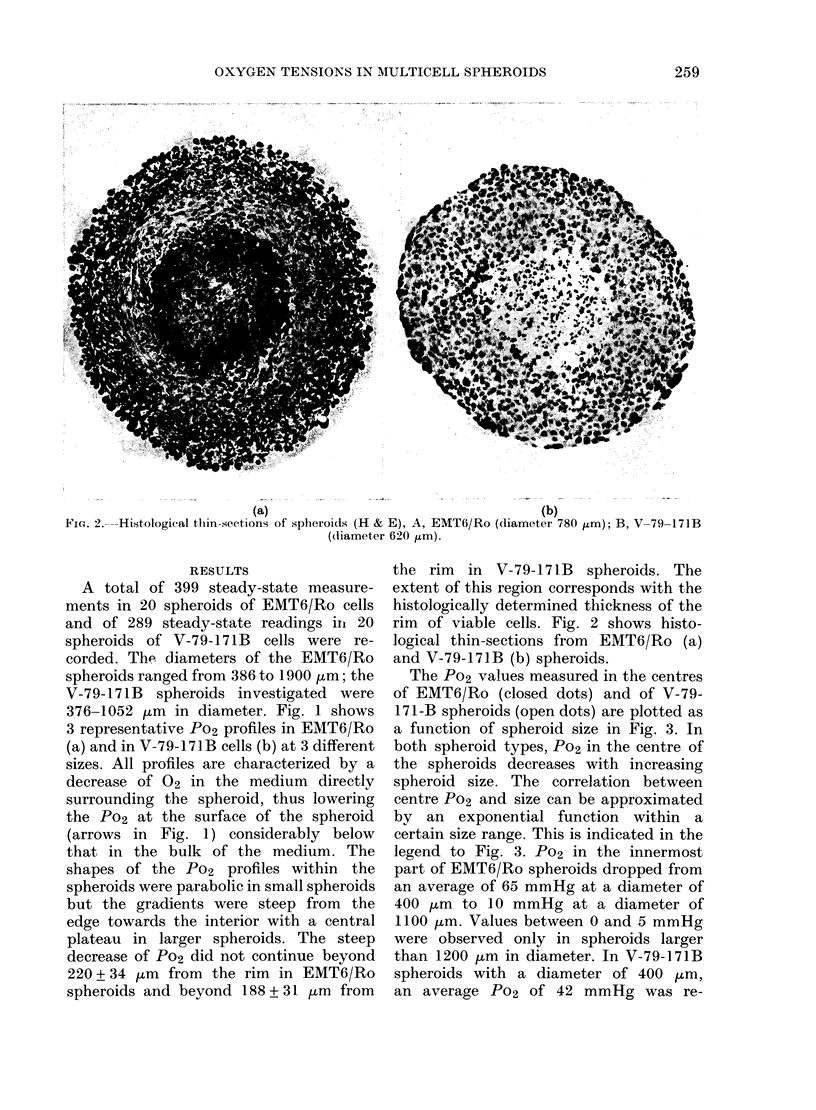
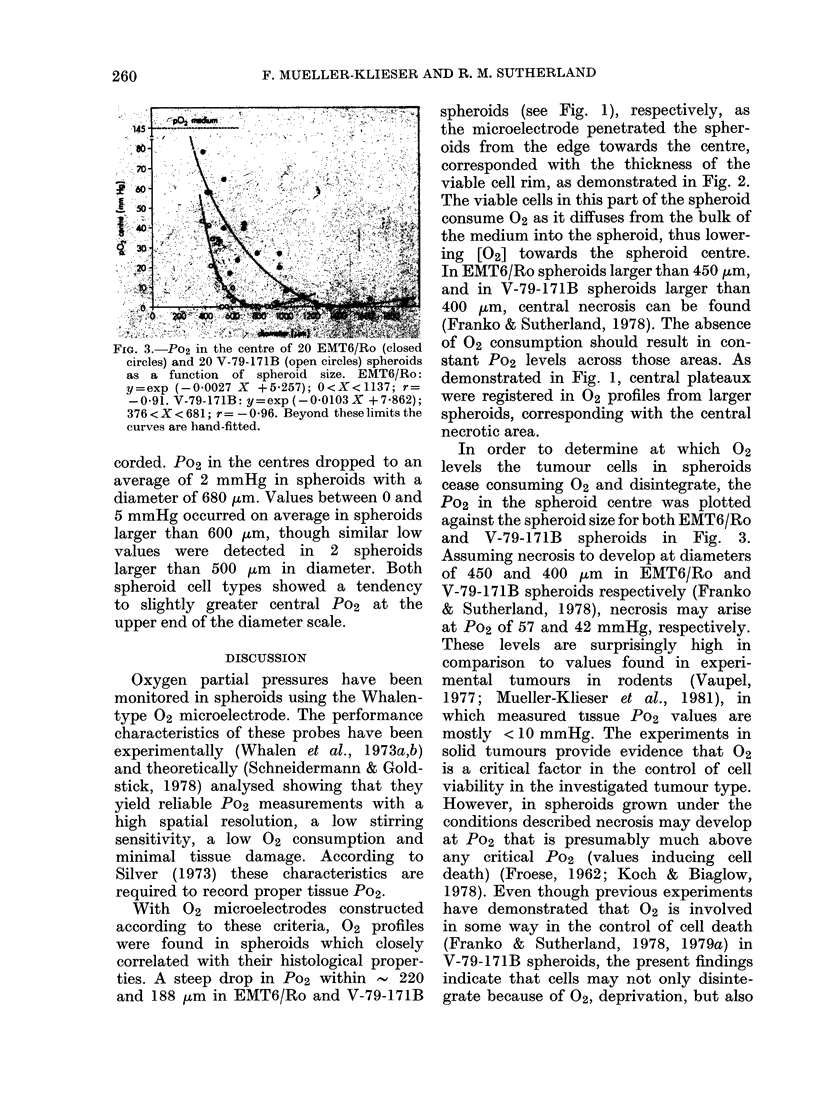
O2 tensions (Po2) were measured with microelectrodes in multicellular spheroids from EMT6/Ro and V-79-171-B cells. The measurements were performed in spheroids kept in flowing growth medium that was equilibrated with 5% CO2 and air at a temperature of 37 degrees C and contained 5.5 mM glucose. The recorded Po2 profiles are characterized by a diffusion-depleted zone surrounding the spheroids and by a steep drop in Po2 within the spheroids over mean distance of 220 and 188 micrometer from the surface of EMT6/Ro and V-79-171B spheroids over mean distance of 220 and 188 micrometer from the surface of EMT6/Ro and V-79-171B spheroids respectively. Smaller spheroid exhibit parabolic Po2 profiles, larger ones show a central plateau. The region of the steep decrease in Po2 corresponds to the thickness of the viable rim: the plateau region is created by the absence of O2 consumption in the central necrotic area. Po2 in the centre of EMT6/Ro spheroids decreased from 66 mmHg at a diameter of 400 micrometer to 13 mmHg at a diameter of 1000 micrometer. Under the present conditions during growth and in the experiments, values below 5 mmHg were recorded only in spheroids 1200 micrometer. Comparably low Po2 was recorded in V-79 spheroids with diameters of 650 micrometer +. In spheroids of this cell type with a diameter of 400 micrometer, Po2 was 42 mmHg. The findings provide evidence that necrosis may arise at average Po2 of 57 and 42 mmHg in EMT6/Ro and V-79-171B spheroids, respectively, grown under the conditions described.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bock P. E., Frieden C. Phosphofructokinase. I. Mechanism of the pH-dependent inactivation and reactivation of the rabbit muscle enzyme. J Biol Chem. 1976 Sep 25;251(18):5630–5636. [PubMed] [Google Scholar]
- Carlsson J., Stålnacke C. G., Acker H., Haji-Karim M., Nilsson S., Larsson B. The influence of oxygen on viability and proliferation in cellular spheroids. Int J Radiat Oncol Biol Phys. 1979 Nov-Dec;5(11-12):2011–2020. doi: 10.1016/0360-3016(79)90953-2. [DOI] [PubMed] [Google Scholar]
- Crabtree H. G. Observations on the carbohydrate metabolism of tumours. Biochem J. 1929;23(3):536–545. doi: 10.1042/bj0230536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FROESE G. The respiration of ascites tumour cells at low oxygen concentrations. Biochim Biophys Acta. 1962 Mar 12;57:509–519. doi: 10.1016/0006-3002(62)91158-7. [DOI] [PubMed] [Google Scholar]
- Franko A. J., Sutherland R. M. Oxygen diffusion distance and development of necrosis in multicell spheroids. Radiat Res. 1979 Sep;79(3):439–453. [PubMed] [Google Scholar]
- Franko A. J., Sutherland R. M. Radiation survival of cells from spheroids grown in different oxygen concentrations. Radiat Res. 1979 Sep;79(3):454–467. [PubMed] [Google Scholar]
- Franko A. J., Sutherland R. M. Rate of death of hypoxic cells in multicell spheroids. Radiat Res. 1978 Dec;76(3):561–572. [PubMed] [Google Scholar]
- Freyer J. P., Sutherland R. M. Selective dissociation and characterization of cells from different regions of multicell tumor spheroids. Cancer Res. 1980 Nov;40(11):3956–3965. [PubMed] [Google Scholar]
- Gosalvez M., Weinhouse S. Control mechanisms of oxygen and glucose utilization in tumours. Adv Exp Med Biol. 1976;75:587–596. doi: 10.1007/978-1-4684-3273-2_69. [DOI] [PubMed] [Google Scholar]
- Kaufman N., Bicher H. I., Hetzel F. W., Brown M. A system for determining the pharmacology of indirect radiation sensitizer drugs on multicellular spheroids. Cancer Clin Trials. 1981;4(2):199–204. [PubMed] [Google Scholar]
- Koch C. J., Biaglow J. E. Respiration of mammalian cells at low concentrations of oxygen: I. Effect of hypoxic-cell radiosensitizing drugs. Br J Cancer Suppl. 1978 Jun;3:163–167. [PMC free article] [PubMed] [Google Scholar]
- Mueller-Klieser W., Vaupel P., Manz R., Schmidseder R. Intracapillary oxyhemoglobin saturation of malignant tumors in humans. Int J Radiat Oncol Biol Phys. 1981 Oct;7(10):1397–1404. doi: 10.1016/0360-3016(81)90036-5. [DOI] [PubMed] [Google Scholar]
- Schneiderman G., Goldstick T. K. Oxygen electrode design criteria and performance characteristics: recessed cathode. J Appl Physiol Respir Environ Exerc Physiol. 1978 Jul;45(1):145–154. doi: 10.1152/jappl.1978.45.1.145. [DOI] [PubMed] [Google Scholar]
- Sutherland R. M., Durand R. E. Hypoxic cells in an in vitro tumour model. Int J Radiat Biol Relat Stud Phys Chem Med. 1973 Mar;23(3):235–246. doi: 10.1080/09553007314550261. [DOI] [PubMed] [Google Scholar]
- Sutherland R. M., Durand R. E. Radiation response of multicell spheroids--an in vitro tumour model. Curr Top Radiat Res Q. 1976 Jan;11(1):87–139. [PubMed] [Google Scholar]
- Sutherland R. M., McCredie J. A., Inch W. R. Growth of multicell spheroids in tissue culture as a model of nodular carcinomas. J Natl Cancer Inst. 1971 Jan;46(1):113–120. [PubMed] [Google Scholar]
- Tannock I. F. Oxygen diffusion and the distribution of cellular radiosensitivity in tumours. Br J Radiol. 1972 Jul;45(535):515–524. doi: 10.1259/0007-1285-45-535-515. [DOI] [PubMed] [Google Scholar]
- Vaupel P. Hypoxia in neoplastic tissue. Microvasc Res. 1977 May;13(3):399–408. doi: 10.1016/0026-2862(77)90106-6. [DOI] [PubMed] [Google Scholar]
- Vaupel P., Manz R., Müller-Klieser W., Grunewald W. A. Intracapillary HbO2 saturation in malignant tumors during normoxia and hyperoxia. Microvasc Res. 1979 Mar;17(2):181–191. doi: 10.1016/0026-2862(79)90405-9. [DOI] [PubMed] [Google Scholar]
- Vaupel P., Thews G. Pathophysiological aspects of glucose uptake by the tumor tissue under various conditions of oxygen and glucose supply. Adv Exp Med Biol. 1976;75:547–553. doi: 10.1007/978-1-4684-3273-2_64. [DOI] [PubMed] [Google Scholar]
- Whalen W. J., Nair P., Ganfield R. A. Measurements of oxygen tension in tissues with a micro oxygen electrode. Microvasc Res. 1973 May;5(3):254–262. doi: 10.1016/0026-2862(73)90035-6. [DOI] [PubMed] [Google Scholar]
- Whalen W. J., Nair P. Intracellular PO2 and its regulation in resting skeletal muscle of the guinea pig. Circ Res. 1967 Sep;21(3):251–261. doi: 10.1161/01.res.21.3.251. [DOI] [PubMed] [Google Scholar]
- Whalen W. J., Savoca J., Nair P. Oxygen tension measurements in carotid body of the cat. Am J Physiol. 1973 Oct;225(4):986–991. doi: 10.1152/ajplegacy.1973.225.4.986. [DOI] [PubMed] [Google Scholar]
- ZWARTOUW H. T., WESTWOOD J. C. Factors affecting growth and glycolysis in tissue culture. Br J Exp Pathol. 1958 Oct;39(5):529–539. [PMC free article] [PubMed] [Google Scholar]