Abstract
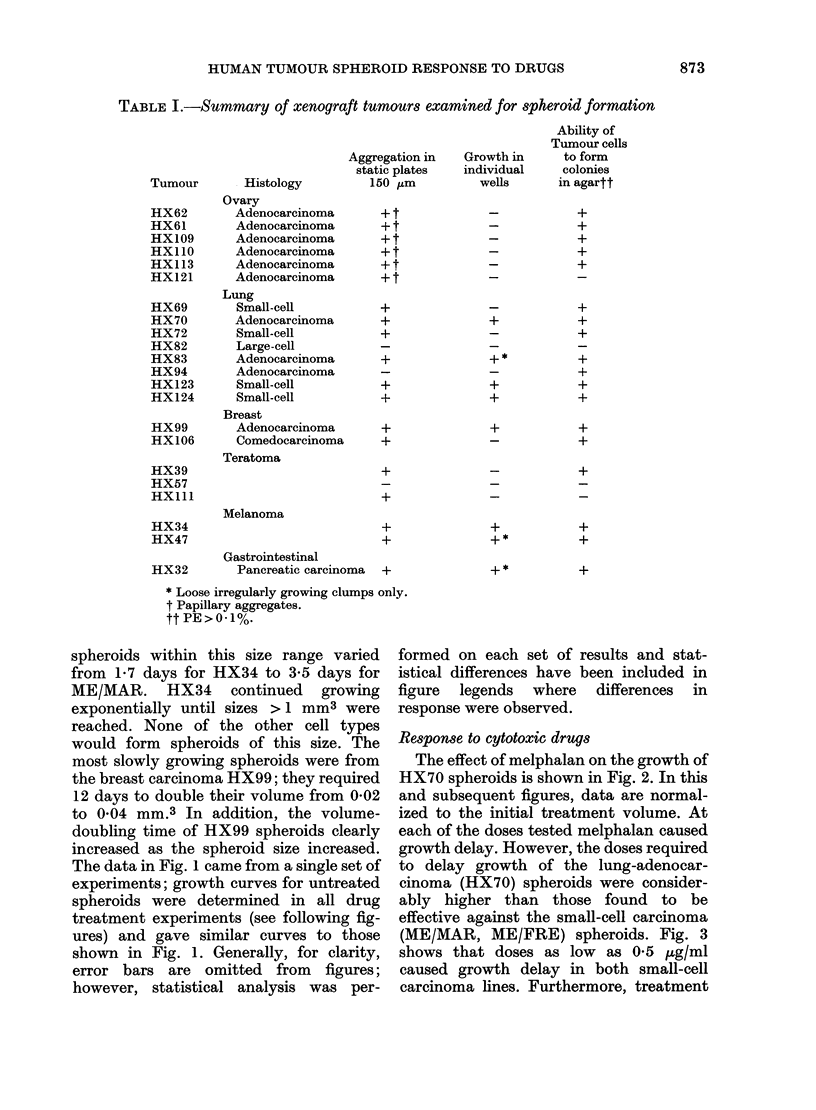
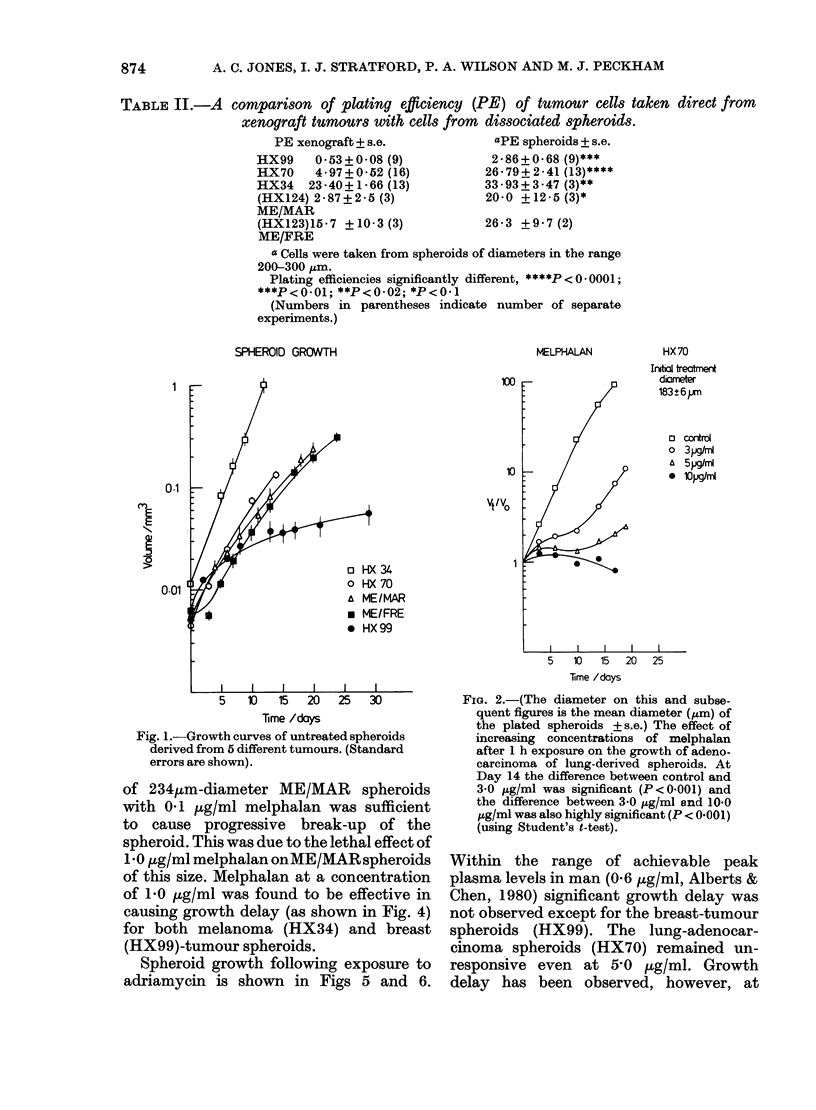
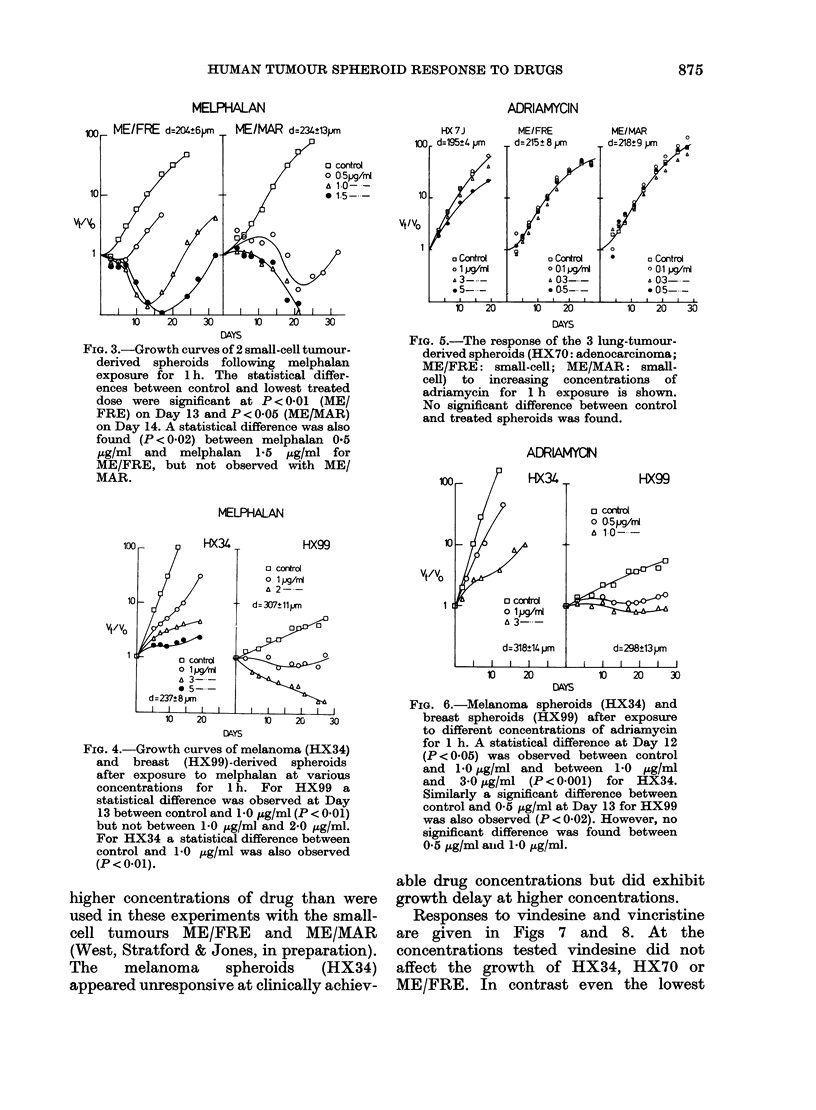
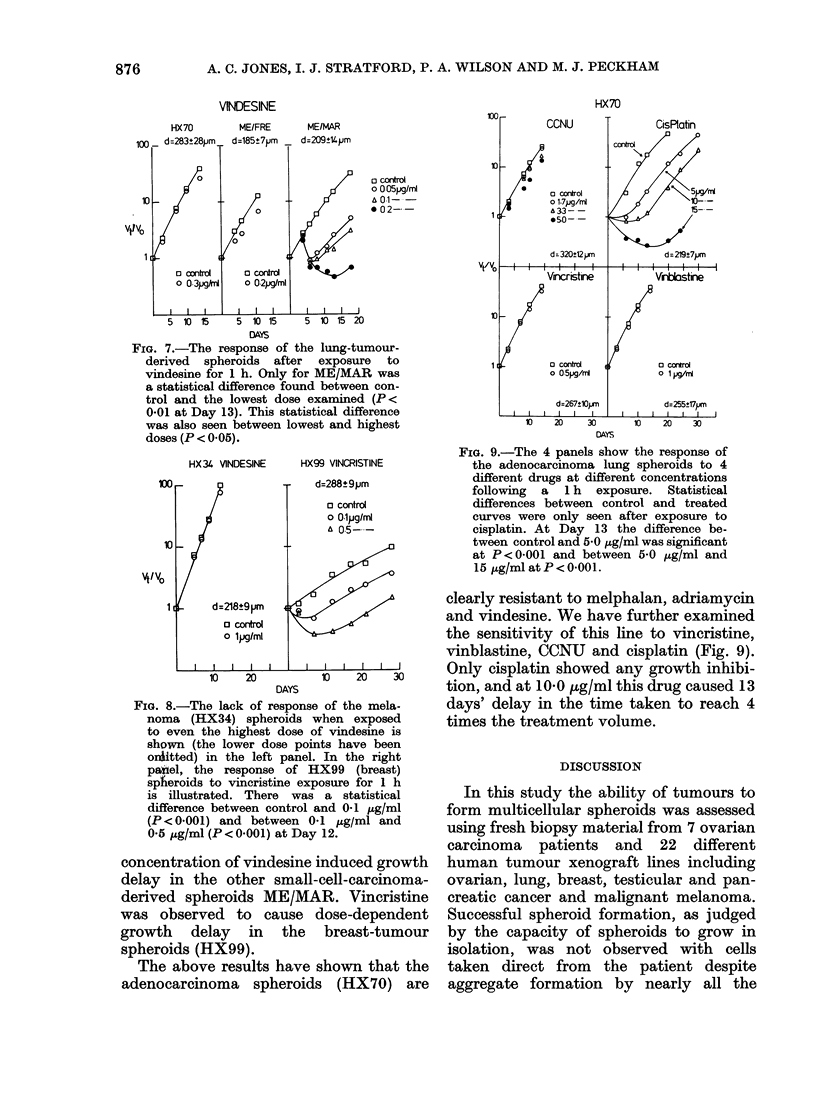
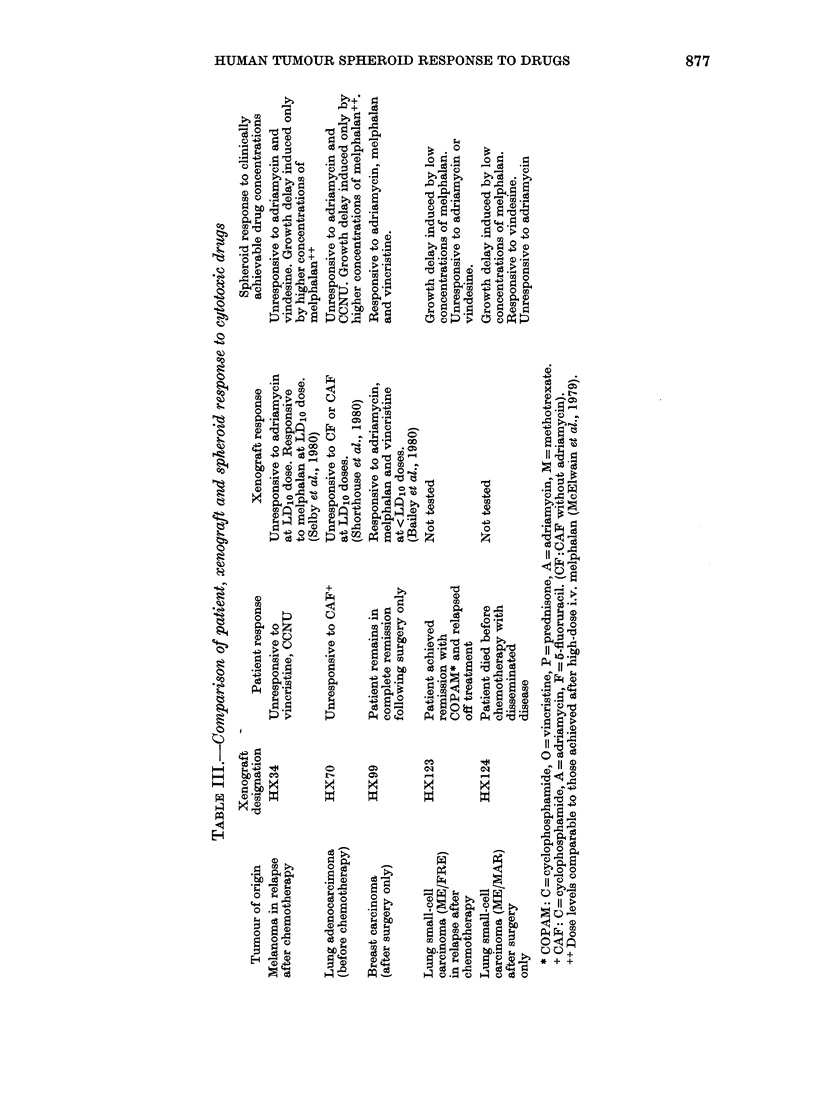
Tumour cells from 7 patients with ovarian carcinoma and from 22 different human tumour xenografts representing a wide range of histological sub-types have been examined for multicellular spheroid forming ability. Spheroid formation was limited to cells derived from xenografts. Of the 22 lines tested, 5 formed spheroids capable of growth in isolation. There was no clear relationship between histological type and spheroid-forming ability. The plating efficiency of tumour cells obtained from spheroids was always greater than for the cells obtained from the dissociated tumour of origin and was in some cases as much as 6-fold greater. Spheroid growth was nearly exponential for 4 cell lines. Volume growth delay was used to investigate the activity of melphalan, adriamycin, the Vinca alkaloids, CCNU and cisplatin. Differences between lines in drug response broadly reflected patient and in vivo xenograft response.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey M. J., Gazet J. C., Smith I. E., Steel G. G. Chemotherapy of human breast-carcinoma xenografts. Br J Cancer. 1980 Oct;42(4):530–536. doi: 10.1038/bjc.1980.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtenay V. D. A soft agar colony assay for Lewis lung tumour and B16 melanoma taken directly from the mouse. Br J Cancer. 1976 Jul;34(1):39–45. doi: 10.1038/bjc.1976.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand R. E., Sutherland R. M. Repair and reoxygenation following irradiation of an in vitro tumor model. Int J Radiat Oncol Biol Phys. 1976 Nov-Dec;1(11-12):1119–1124. doi: 10.1016/0360-3016(76)90084-5. [DOI] [PubMed] [Google Scholar]
- Ellison M. L., Hillyard C. J., Bloomfield G. A., Rees L. H., Coombes R. C., Neville A. M. Ectopic hormone production by bronchial carcinomas in culture. Clin Endocrinol (Oxf) 1976;5 (Suppl):397S–406S. doi: 10.1111/j.1365-2265.1976.tb03848.x. [DOI] [PubMed] [Google Scholar]
- Haji-Karim M., Carlsson J. Proliferation and viability in cellular spheroids of human origin. Cancer Res. 1978 May;38(5):1457–1464. [PubMed] [Google Scholar]
- Nowak K., Peckham M. J., Steel G. G. Variation in response of xenografts of colo-rectal carcinoma to chemotherapy. Br J Cancer. 1978 Apr;37(4):576–584. doi: 10.1038/bjc.1978.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourreau-Schneider N., Malaise E. P. Relationship between surviving fractions using the colony methods, the LD50, and the growth delay after irradiation of human melanoma cells grown as multicellular spheroids. Radiat Res. 1981 Feb;85(2):321–332. [PubMed] [Google Scholar]
- Selby P. J., Courtenay V. D., McElwain T. J., Peckham M. J., Steel G. G. Colony growth and clonogenic cell survival in human melanoma xenografts treated with chemotherapy. Br J Cancer. 1980 Sep;42(3):438–447. doi: 10.1038/bjc.1980.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorthouse A. J., Smyth J. F., Steel G. G., Ellison M., Mills J., Peckham M. J. The human tumour xenograft--a valid model in experimental chemotherapy? Br J Surg. 1980 Oct;67(10):715–722. doi: 10.1002/bjs.1800671011. [DOI] [PubMed] [Google Scholar]
- Steel G. G., Courtenay V. D., Rostom A. Y. Improved immune-suppression techniques for the exongrafting of human tumours. Br J Cancer. 1978 Feb;37(2):224–230. doi: 10.1038/bjc.1978.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland R. M., Eddy H. A., Bareham B., Reich K., Vanantwerp D. Resistance to adriamycin in multicellular spheroids. Int J Radiat Oncol Biol Phys. 1979 Aug;5(8):1225–1230. doi: 10.1016/0360-3016(79)90643-6. [DOI] [PubMed] [Google Scholar]
- Sutherland R. M., McCredie J. A., Inch W. R. Growth of multicell spheroids in tissue culture as a model of nodular carcinomas. J Natl Cancer Inst. 1971 Jan;46(1):113–120. [PubMed] [Google Scholar]
- Twentyman P. R. Response to chemotherapy of EMT6 spheroids as measured by growth delay and cell survival. Br J Cancer. 1980 Aug;42(2):297–304. doi: 10.1038/bjc.1980.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West G. W., Weichselbaum R., Little J. B. Limited penetration of methotrexate into human osteosarcoma spheroids as a proposed model for solid tumor resistance to adjuvant chemotherapy. Cancer Res. 1980 Oct;40(10):3665–3668. [PubMed] [Google Scholar]
- Wibe E. Resistance to vincristine of human cells grown as multicellular spheroids. Br J Cancer. 1980 Dec;42(6):937–941. doi: 10.1038/bjc.1980.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson W. R., Whitmore G. F., Hill R. P. Activity of 4'-(9-acridinylamino)methanesulfon-m-anisidide against Chinese hamster cells in multicellular spheroids. Cancer Res. 1981 Jul;41(7):2817–2822. [PubMed] [Google Scholar]
- Yuhas J. M., Li A. P. Growth fraction as the major determinant of multicellular tumor spheroid growth rates. Cancer Res. 1978 Jun;38(6):1528–1532. [PubMed] [Google Scholar]
- Yuhas J. M., Tarleton A. E., Culo F. Tumor line dependent interactions of irradiation and cis-diamminedichloroplatinum in the multicellular tumor spheroid system. Int J Radiat Oncol Biol Phys. 1979 Aug;5(8):1373–1375. doi: 10.1016/0360-3016(79)90673-4. [DOI] [PubMed] [Google Scholar]
- Yuhas J. M., Tarleton A. E., Harman J. G. In vitro analysis of the response of multicellular tumor spheroids exposed to chemotherapeutic agents in vitro or in vivo. Cancer Res. 1978 Nov;38(11 Pt 1):3595–3598. [PubMed] [Google Scholar]
- Yuhas J. M., Tarleton A. E., Molzen K. B. Multicellular tumor spheroid formation by breast cancer cells isolated from different sites. Cancer Res. 1978 Aug;38(8):2486–2491. [PubMed] [Google Scholar]


