Abstract
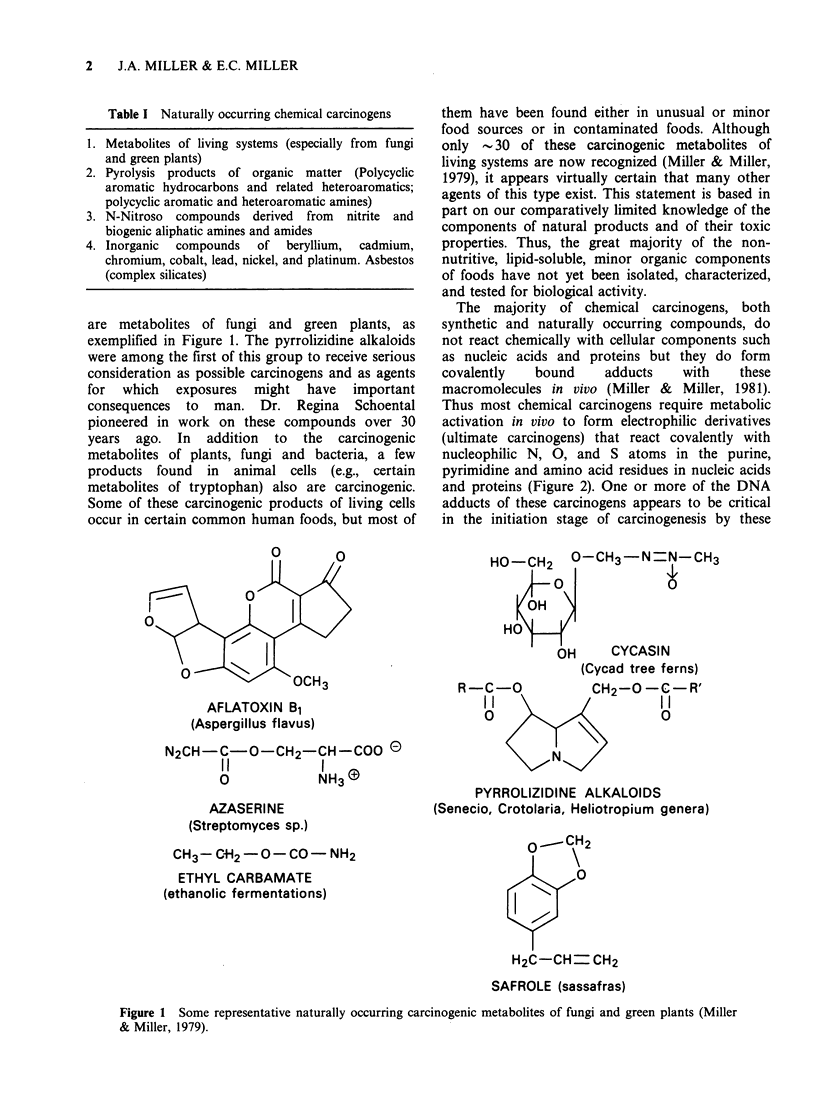
A small (approximately 30) but varied group of organic and inorganic compounds appear to be carcinogenic in both humans and experimental animals. A much larger number and wider variety of chemical carcinogens, primarily synthetic organic compounds, are known for experimental animals. These agents include a small (approximately 30) and varied group of metabolites of green plants and fungi. Many more of these carcinogens must exist in the living world. As with the synthetic carcinogens, the majority of these naturally occurring carcinogens are procarcinogens that require metabolic conversion into reactive electrophilic and mutagenic ultimate carcinogens. These strong electrophiles combine covalently and non-enzymatically with nucleophilic sites in DNAs, RNAs, proteins, and small molecules in target tissues. One or more of the DNA adducts appear to initiate carcinogenesis in an irreversible manner. The subsequent promotion step leading to gross tumours may be completed by further administration of carcinogen or by treatment with non-carcinogenic promoters. Roles for the RNA and protein adducts in the carcinogenic process have not been excluded. Recent data on the metabolic activation and reactivity in vivo of the naturally occurring carcinogens ethyl carbamate and certain of the alkenylbenzene spice flavours are illustrative of these principles. These agents can initiate the carcinogenic process in male mouse liver with small doses given prior to weaning. Subsequent growth of the liver and male hormonal factors appear to function as promoters leading to gross hepatic tumors after one year. Reactive electrophilic metabolites of ethyl carbamate and of safrole and estragole and their nucleic acid adducts formed during initiation in mouse liver have been characterized.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrio J. R., Secrist J. A., 3rd, Leonard N. J. Fluorescent adenosine and cytidine derivatives. Biochem Biophys Res Commun. 1972 Jan 31;46(2):597–604. doi: 10.1016/s0006-291x(72)80181-5. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Oncogenes. Sci Am. 1982 Mar;246(3):80–92. doi: 10.1038/scientificamerican0382-80. [DOI] [PubMed] [Google Scholar]
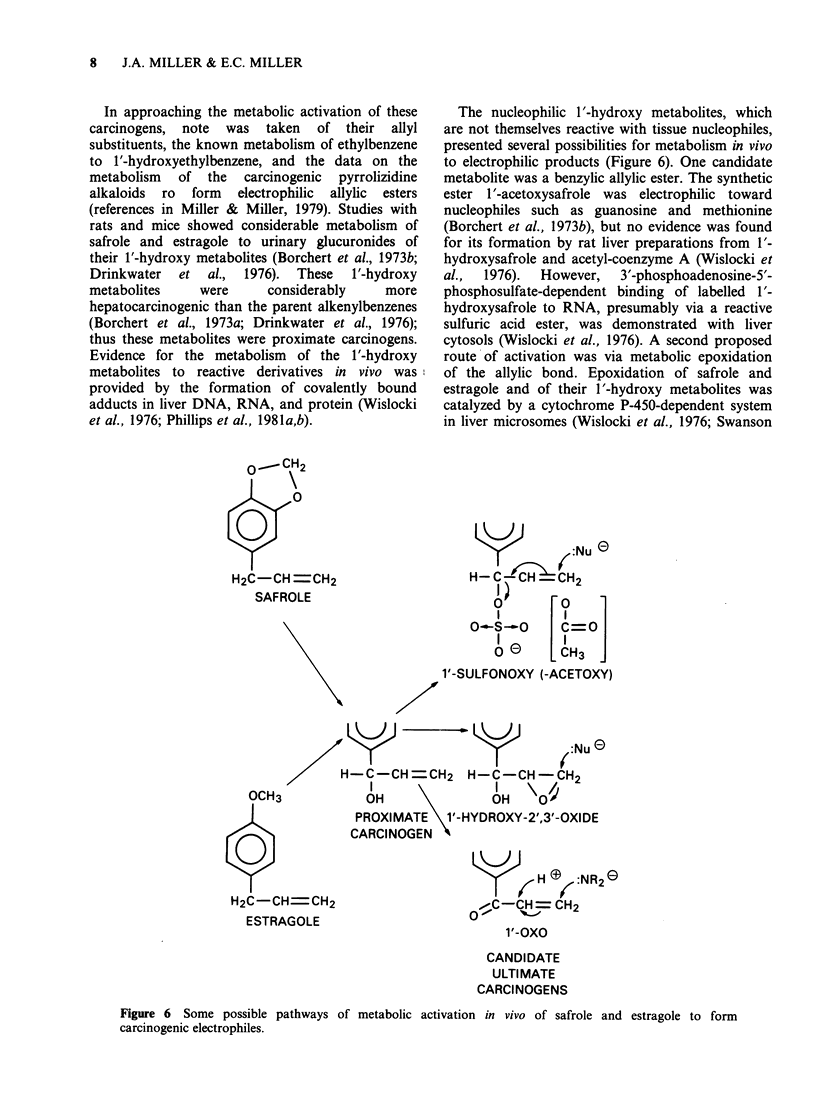
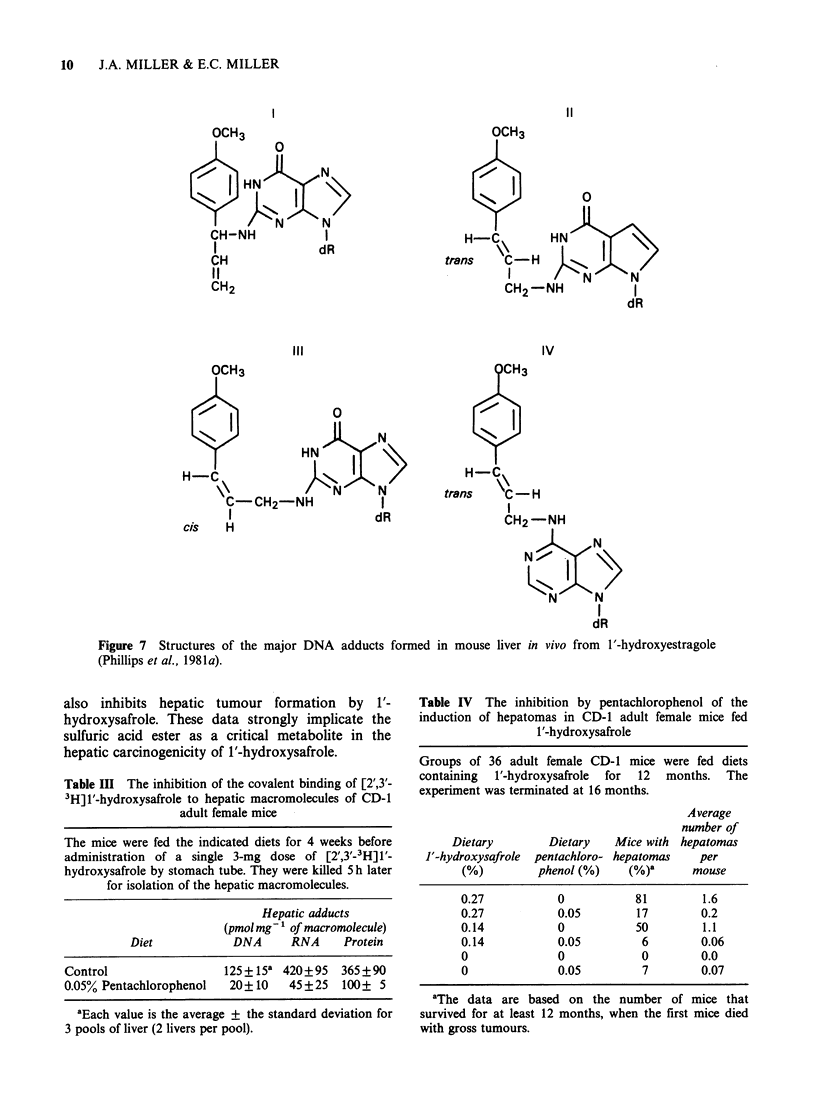
- Borchert P., Miller J. A., Miller E. C., Shires T. K. 1'-Hydroxysafrole, a proximate carcinogenic metabolite of safrole in the rat and mouse. Cancer Res. 1973 Mar;33(3):590–600. [PubMed] [Google Scholar]
- Borchert P., Wislocki P. G., Miller J. A., Miller E. C. The metabolism of the naturally occurring hepatocarcinogen safrole to 1'-hydroxysafrole and the electrophilic reactivity of 1'-acetoxysafrole. Cancer Res. 1973 Mar;33(3):575–589. [PubMed] [Google Scholar]
- Dahl G. A., Miller E. C., Miller J. A. Comparative carcinogenicities and mutagenicities of vinyl carbamate, ethyl carbamate, and ethyl N-hydroxycarbamate. Cancer Res. 1980 Apr;40(4):1194–1203. [PubMed] [Google Scholar]
- Dahl G. A., Miller J. A., Miller E. C. Vinyl carbamate as a promutagen and a more carcinogenic analog of ethyl carbamate. Cancer Res. 1978 Nov;38(11 Pt 1):3793–3804. [PubMed] [Google Scholar]
- Drinkwater N. R., Miller E. C., Miller J. A., Pitot H. C. Hepatocarcinogenicity of estragole (1-allyl-4-methoxybenzene) and 1'-hydroxyestragole in the mouse and mutagenicity of 1'-acetoxyestragole in bacteria. J Natl Cancer Inst. 1976 Dec;57(6):1323–1331. doi: 10.1093/jnci/57.6.1323. [DOI] [PubMed] [Google Scholar]
- Laib R. J., Bolt H. M. Alkylation of RNA by vinyl chloride metabolites in vitro and in vivo: formation of 1-N(6)-etheno-adenosine. Toxicology. 1977 Oct;8(2):185–195. doi: 10.1016/0300-483x(77)90007-5. [DOI] [PubMed] [Google Scholar]
- Laib R. J., Bolt H. M. Formation of 3,N4-ethenocytidine moieties in RNA by vinyl chloride metabolities in vitro and in vivo. Arch Toxicol. 1978 Jan 25;39(3):235–240. doi: 10.1007/BF00368232. [DOI] [PubMed] [Google Scholar]
- Laib R. J., Gwinner L. M., Bolt H. M. DNA alkylation by vinyl chloride metabolites: etheno derivatives or 7-alkylation of guanine? Chem Biol Interact. 1981 Oct;37(1-2):219–231. doi: 10.1016/0009-2797(81)90179-4. [DOI] [PubMed] [Google Scholar]
- Lawson T. A., Pound A. W. The interaction of carbon-14-labelled alkyl carbamates, labelled in the alkyl and carbonyl positions, with DNA in vivo. Chem Biol Interact. 1973 Feb;6(2):99–105. doi: 10.1016/0009-2797(73)90076-8. [DOI] [PubMed] [Google Scholar]
- Meerman J. H., Beland F. A., Mulder G. J. Role of sulfation in the formation of DNA adducts from N-hydroxy-2-acetylaminofluorene in rat liver in vivo. Inhibition of N-acetylated aminofluorene adduct formation by pentachlorophenol. Carcinogenesis. 1981;2(5):413–416. doi: 10.1093/carcin/2.5.413. [DOI] [PubMed] [Google Scholar]
- Meerman J. H., van Doorn A. B., Mulder G. J. Inhibition of sulfate conjugation of N-hydroxy-2-acetylaminofluorene in isolated perfused rat liver and in the rat in vivo by pentachlorophenol and low sulfate. Cancer Res. 1980 Oct;40(10):3772–3779. [PubMed] [Google Scholar]
- Miller E. C., Miller J. A. Searches for ultimate chemical carcinogens and their reactions with cellular macromolecules. Cancer. 1981 May 15;47(10):2327–2345. doi: 10.1002/1097-0142(19810515)47:10<2327::aid-cncr2820471003>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Miller E. C., Swanson A. B., Phillips D. H., Fletcher T. L., Liem A., Miller J. A. Structure-activity studies of the carcinogenicities in the mouse and rat of some naturally occurring and synthetic alkenylbenzene derivatives related to safrole and estragole. Cancer Res. 1983 Mar;43(3):1124–1134. [PubMed] [Google Scholar]
- Mirvish S. S. The carcinogenic action and metabolism of urethan and N-hydroxyurethan. Adv Cancer Res. 1968;11:1–42. doi: 10.1016/s0065-230x(08)60386-3. [DOI] [PubMed] [Google Scholar]
- Newmark P. Still more about oncogenes. Nature. 1983 Jan 13;301(5896):111–111. doi: 10.1038/301111a0. [DOI] [PubMed] [Google Scholar]
- Nomura T. Urethan (ethyl carbamate) as a cosolvent of drugs commonly used parenterally in humans. Cancer Res. 1975 Oct;35(10):2895–2899. [PubMed] [Google Scholar]
- Osterman-Golkar S., Hultmark D., Segerbäck D., Calleman C. J., Göthe R., Ehrenberg L., Wachtmeister C. A. Alkylation of DNA and proteins in mice exposed to vinyl chloride. Biochem Biophys Res Commun. 1976 May 23;76(2):259–266. doi: 10.1016/0006-291x(77)90720-3. [DOI] [PubMed] [Google Scholar]
- Oswald E. O., Fishbein L., Corbett B. J., Walker M. P. Identification of tertiary aminomethylenedioxypropiophenones as urinary metabolites of safrole in the rat and guinea pig. Biochim Biophys Acta. 1971 Feb 23;230(2):237–247. doi: 10.1016/0304-4165(71)90208-x. [DOI] [PubMed] [Google Scholar]
- Ough C. S. Ethylcarbamate in fermented beverages and foods. I. Naturally occurring ethylcarbamate. J Agric Food Chem. 1976 Mar-Apr;24(2):323–328. doi: 10.1021/jf60204a033. [DOI] [PubMed] [Google Scholar]
- Phillips D. H., Miller J. A., Miller E. C., Adams B. N2 atom of guanine and N6 atom of adenine residues as sites for covalent binding of metabolically activated 1'-hydroxysafrole to mouse liver DNA in vivo. Cancer Res. 1981 Jul;41(7):2664–2671. [PubMed] [Google Scholar]
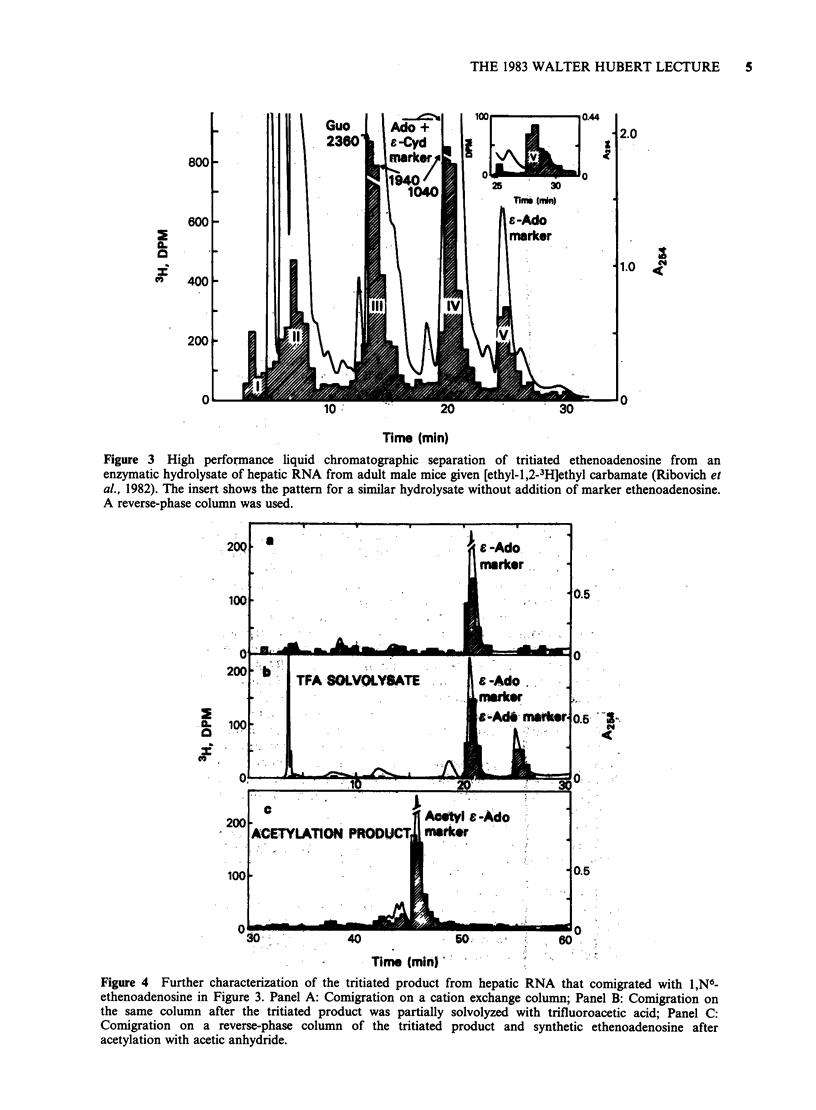
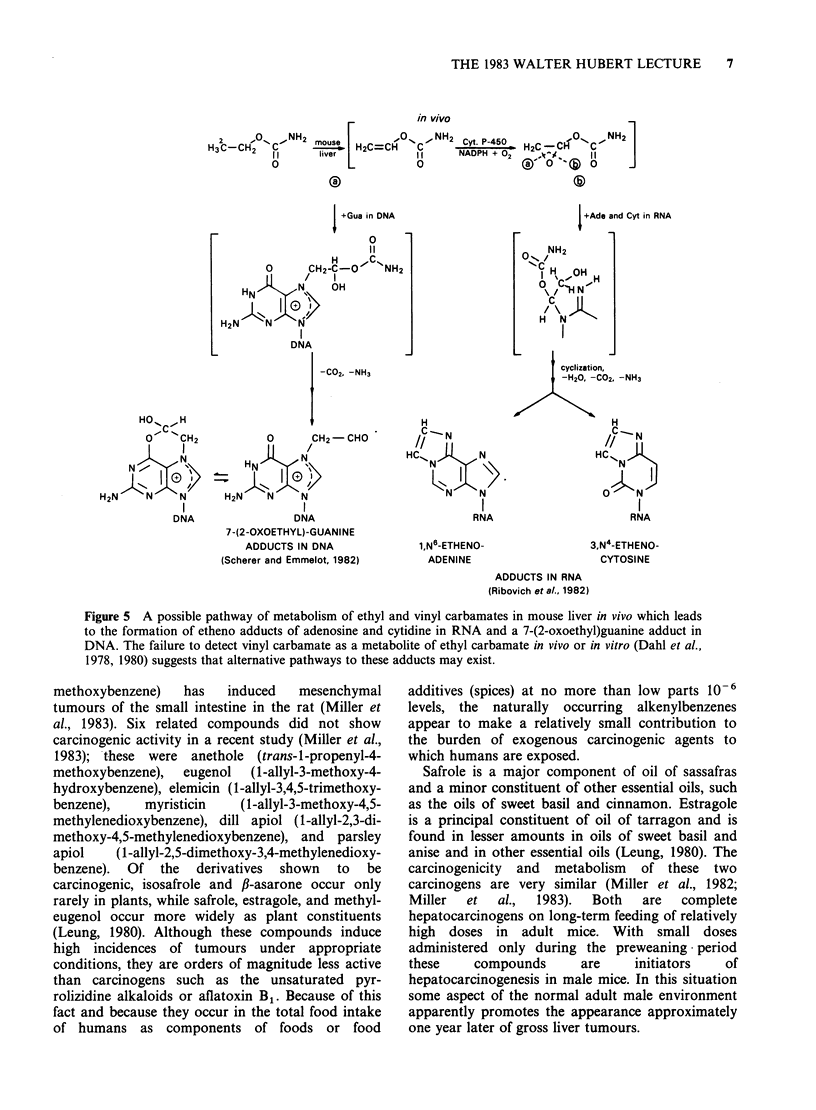
- Ribovich M. L., Miller J. A., Miller E. C., Timmins L. G. Labeled 1,N6-ethenoadenosine and 3,N4-ethenocytidine in hepatic RNA of mice given[ethyl-1,2(-3)H or ethyl-1(-14)C]ethyl carbamate (urethan). Carcinogenesis. 1982;3(5):539–546. doi: 10.1093/carcin/3.5.539. [DOI] [PubMed] [Google Scholar]
- Rigby P. W. The oncogenic circle closes. Nature. 1982 Jun 10;297(5866):451–453. doi: 10.1038/297451a0. [DOI] [PubMed] [Google Scholar]
- Schaaper R. M., Glickman B. W., Loeb L. A. Role of depurination in mutagenesis by chemical carcinogens. Cancer Res. 1982 Sep;42(9):3480–3485. [PubMed] [Google Scholar]
- Scherer E., Steward A. P., Emmelot P. Formation of precancerous islands in rat liver and modification of DNA by ethyl carbamate: implications for its metabolism. Dev Toxicol Environ Sci. 1980;8:249–254. [PubMed] [Google Scholar]
- Scherer E., Van der Laken C. J., Gwinner L. M., Laib R. J., Emmelot P. Modification of deoxyguanosine by chloroethylene oxide. Carcinogenesis. 1981;2(7):671–677. doi: 10.1093/carcin/2.7.671. [DOI] [PubMed] [Google Scholar]
- Shimkin M. B., Wieder R., McDonough M., Fishbein L., Swern D. Lung tumor response in strain A mice as a quantitative bioassay of carcinogenic activity of some carbamates and aziridines. Cancer Res. 1969 Dec;29(12):2184–2190. [PubMed] [Google Scholar]
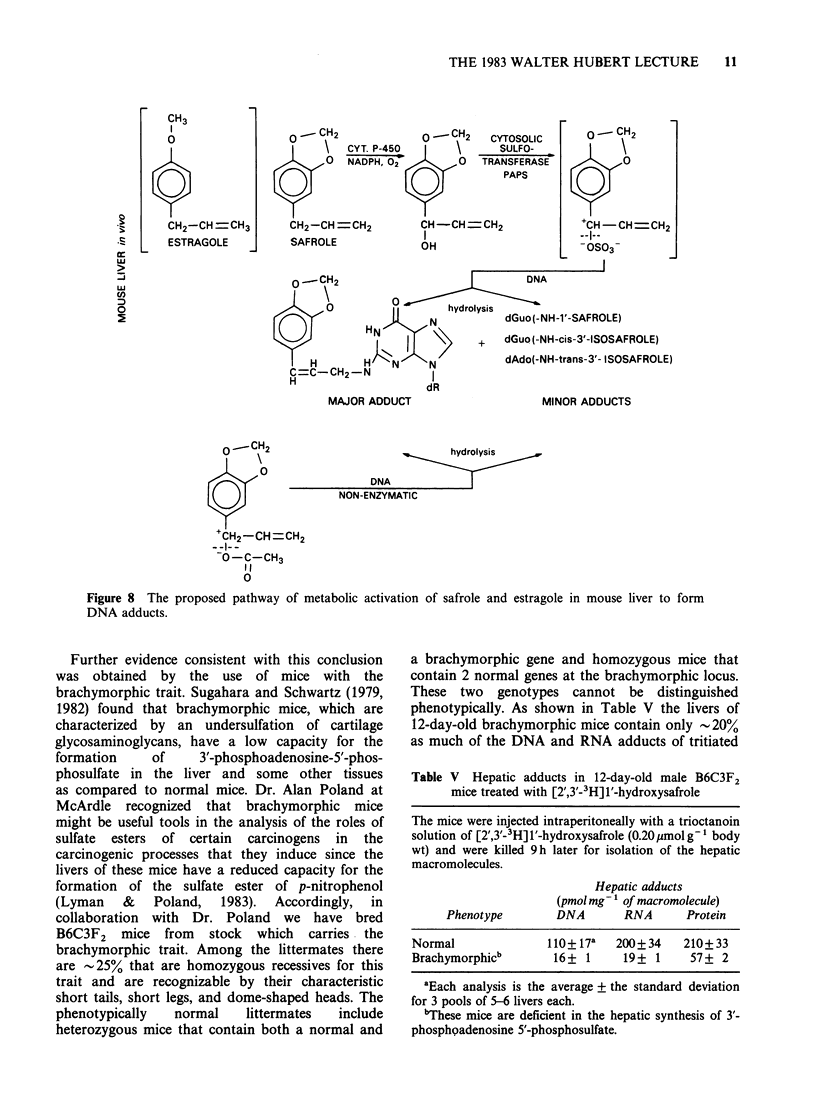
- Sugahara K., Schwartz N. B. Defect in 3'-phosphoadenosine 5'-phosphosulfate formation in brachymorphic mice. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6615–6618. doi: 10.1073/pnas.76.12.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugahara K., Schwartz N. B. Defect in 3'-phosphoadenosine 5'-phosphosulfate synthesis in brachymorphic mice. II. Tissue distribution of the defect. Arch Biochem Biophys. 1982 Apr 1;214(2):602–609. doi: 10.1016/0003-9861(82)90065-0. [DOI] [PubMed] [Google Scholar]
- Swanson A. B., Chambliss D. D., Blomquist J. C., Miller E. C., Miller J. A. The mutagenicities of safrole, estragole, eugenol, trans-anethole, and some of their known or possible metabolites for Salmonella typhimurium mutants. Mutat Res. 1979 Apr;60(2):143–153. doi: 10.1016/0027-5107(79)90178-7. [DOI] [PubMed] [Google Scholar]
- Swanson A. B., Miller E. C., Miller J. A. The side-chain epoxidation and hydroxylation of the hepatocarcinogens safrole and estragole and some related compounds by rat and mouse liver microsomes. Biochim Biophys Acta. 1981 Apr 3;673(4):504–516. doi: 10.1016/0304-4165(81)90482-7. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. Fewer and fewer oncogenes. Cell. 1982 Aug;30(1):3–4. doi: 10.1016/0092-8674(82)90003-4. [DOI] [PubMed] [Google Scholar]
- Weiss R. The myc oncogene in man and birds. Nature. 1982 Sep 2;299(5878):9–10. doi: 10.1038/299009a0. [DOI] [PubMed] [Google Scholar]
- Wislocki P. G., Borchert P., Miller J. A., Miller E. C. The metabolic activation of the carcinogen 1'-hydroxysafrole in vivo and in vitro and the electrophilic reactivities of possible ultimate carcinogens. Cancer Res. 1976 May;36(5):1686–1695. [PubMed] [Google Scholar]
- Wislocki P. G., Miller E. C., Miller J. A., McCoy E. C., Rosenkranz H. S. Carcinogenic and mutagenic activities of safrole, 1'-hydroxysafrole, and some known or possible metabolites. Cancer Res. 1977 Jun;37(6):1883–1891. [PubMed] [Google Scholar]
- Zurlo J., Curphey T. J., Hiley R., Longnecker D. S. Identification of 7-carboxymethylguanine in DNA from pancreatic acinar cells exposed to azaserine. Cancer Res. 1982 Apr;42(4):1286–1288. [PubMed] [Google Scholar]


